Chapter 2: Atoms and Molecules
Lecture Video: Atoms and Molecules
Lecture Slides: Atoms and Molecules
Study Guide: Atoms and Molecules
Lab: Atoms and Molecules
Biology is studied across a wide range of scales, and at the smallest scale biology is nothing more than the interactions of atoms and molecules. While organisms are composed of cells, cells are nothing more than complex chemical interactions.
The Atom
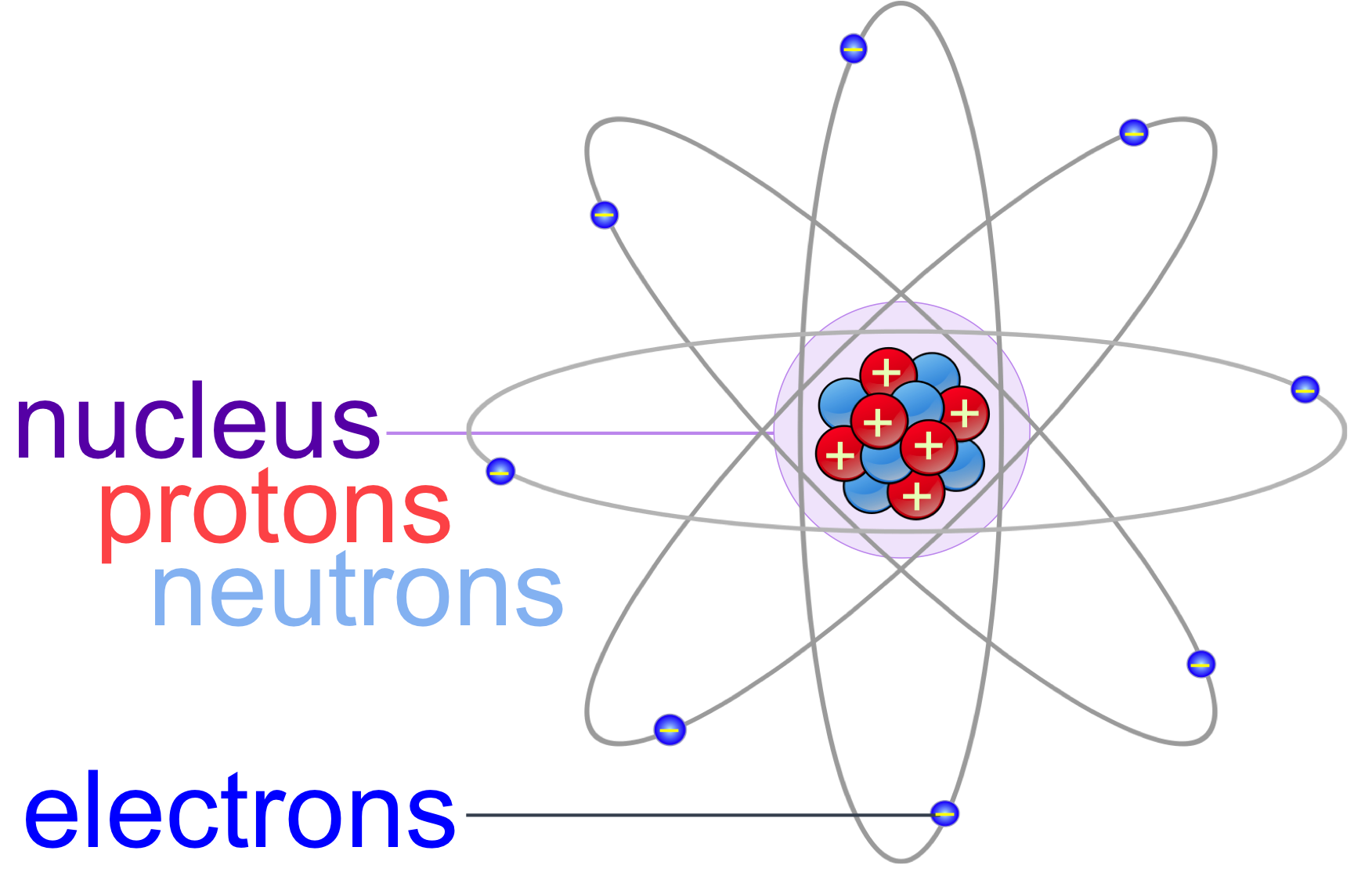
Figure 1. Atoms are the fundamental unit of matter and composed of three subatomic particle: protons, neutrons and electrons. Most of the mass of an atom is within the nucleus, containing the positively charged protons and neutrally charged neutrons. Negatively charged electrons orbit the nucleus, and are responsible for chemical bonding.
Chemistry is the study of matter. Matter is defined as physical substances, which contain mass and take up space. The fundamental unit of matter is the atom, meaning this is the smallest particle of matter that has the properties of that substance. Atoms are composed three subatomic particles: protons, neutrons and electrons (fig. 1). The nucleus of an atom is composed of protons and neutrons within the center of the atom. Different elements have different numbers of protons, which is equal to an element’s atomic number – the whole number found in the periodic table. Protons have a positive electrical charge and neutrons have a neutral electrical charge. Electrons are negatively charged subatomic particles bound to the nucleus by their electromagnetic attraction to the positively charged protons. In contrast, a force 100 times stronger than electromagnetic attraction, known as the nuclear force, overwhelms the repulsive electromagnetic forces of the protons, and binds protons and neutrons together, making the nucleus extremely stable.
The idea of the atom originated in ancient Greece, and atoms were long thought to be perfect homogenous spheres, similar to billiard balls, incapable of being cut into smaller particles. Further investigations beginning in the 19th century turned this idea on its head. The nucleus contains nearly all (99.94%) of its mass, but takes up an extremely small amount of its volume. Though there is variation among the different elements, the nucleus of an atom is approximately 1/100,000th the volume of an atom. In other words, if the nucleus is about the size of a marble, the outer edge of the atom would be length of a football field away and the volume of that atom would take up the space of an entire coliseum. Within that remaining volume are one or more electrons, which (relative to protons and neutrons) and virtually massless, though not completely. What this means is that every thing you can touch is made up almost completely of empty space, indicating that the nucleus, containing nearly all the mass of the atom, is extremely dense. For example, if the human body were an atom, nearly all the mass of the body would be in a nucleus taking up about the amount of space as a drop of water.
Electrons are negatively charged, nearly massless subatomic particles that are bound to the nucleus by their electromagnetic attraction to the positively charged protons. If an atom has same number of protons and electrons, it is neutrally charged as the electrical charges cancel each other out. More electrons than protons result in a negative charge; fewer electrons than protons give an atom a positive charge. Charged atoms are known as ions.
Figure 2. Periodic Table of Elements.
Calculating Subatomic Particles
Figure 3. Calculating subatomic particles. The atomic number represents the number of protons of an element and the number of electrons when the atom is not in a chemical bond. Neutrons of the most common isotopes can be calculated using the atomic mass.
Using the periodic table of the elements (fig. 2), we can calculate number of protons and electrons in a neutrally charged atom, as well as the average number of neutrons. Let’s use carbon as an example. Carbon is element 6, located in the second row (or period as it is called in the periodic table). The atomic number of an element (fig. 3) is the large whole number found in the element’s cell of the periodic table, which indicates the number of that element’s protons. The atomic number for carbon is 6. The atomic number also indicates the number of electrons the atom will have in a neutral state. The number of neutrons can also be calculated using the atomic mass, which is denoted in the element’s cell of the periodic table as a decimal number (fig. 3). The atomic mass of carbon is 12.011. Since the electrons are virtually massless, the atomic mass is housed almost entirely within the nucleus. Subatomic particles are so small that physicists developed a unit of measurement specifically to discuss them, the atomic mass unit. While the mass of protons (1.007amu) and neutrons (1.0009amu) are different, they are extremely similar. For our purposes, we can round the atomic mass units for protons and neutrons to 1. In contrast, the mass of an electron is 0.0005 amu, two thousand times lighter than protons and neutrons – virtually massless.
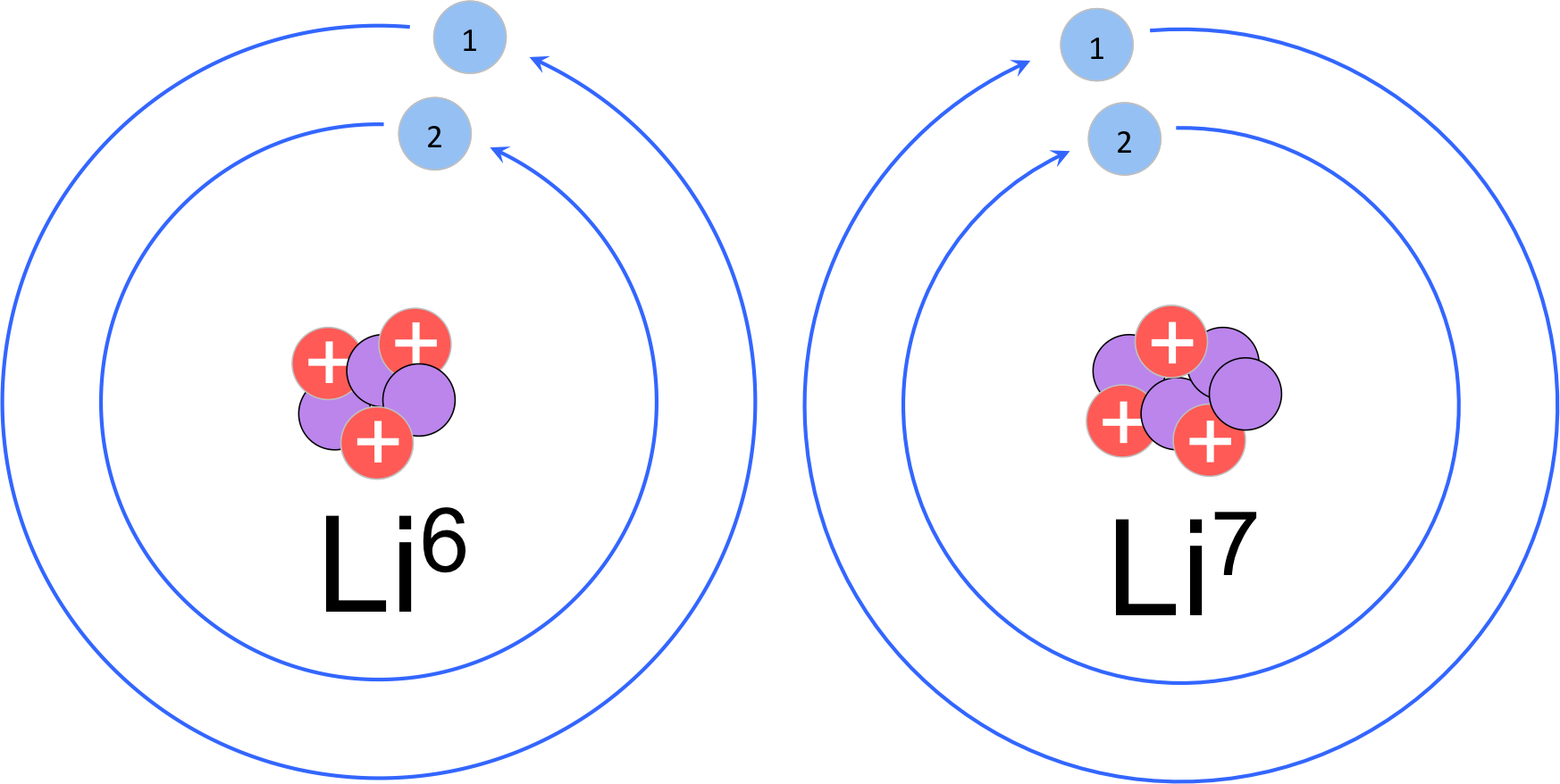
Figure 4 Isotopes of lithium. While elements are defined by the number of protons, elements can vary in the number of neutrons. In this example, lithium-6 has 3 neutrons and lithium-7 has 4 neutrons. Both isotopes have three electrons in a neutral state with two non-reactive electrons in the inner shell, and one reactive electron in the valence shell.
To calculate the number of neutrons of an element, we refer to the periodic table. Since the atomic mass of protons and neutrons are essentially one and the electrons are virtually massless, the atomic mass of an element is equal the number of protons plus the number of neutrons. Since the atomic number is the number of protons, the number of neutrons is equal to the atomic mass minus the atomic number. You’ll notice this will not give you a whole number, and an atom cannot have partial neutrons. While the number of protons defines an element, elements can vary in the number of neutrons contained in the nucleus. Elements with different numbers of neutrons are known as isotopes (fig. 4). The atomic mass is the weighted average of naturally occurring isotopes. Considering carbon with an atomic mass of 12.01, the most common isotope of carbon is carbon-12, which contains 6 protons and 6 electrons. To calculate the neutrons of the most common isotopes, simply round the atomic mass and subtract the number of protons, giving carbon-12 six neutrons. Consider lithium (atomic number 3 & atomic mass 6.941). The most common isotope is lithium-7, with 3 protons and 4 neutrons. Lithium-6 is another isotope of lithium with 3 protons and 3 neutrons, but only naturally occurring about 7% in samples, whereas lithium-7 is much more common (about 93% of the samples).
The Rutherford-Bohr Model of the Atom
Figure 5. Rutherford-Bohr model of a hydrogen atom. An electron orbits a proton in a discrete orbit.
In 1913, Neils Bohr and Ernest Rutherford proposed a model of the hydrogen atom in which negatively charged electrons travel in circular orbits around the positively charged nucleus in a discrete orbit. The Rutherford-Bohr atomic model, generally simplified as the Bohr model (fig. 5), is useful in understanding how electrons orbit the nucleus for other elements and how atoms interact with each other to form chemical bonds. In this model, electrons orbit the nucleus at a specific distance, similar to planets around the sun. Electrons are assigned to different electron shells (fig. 6), or more precisely energy levels, in which electrons were filled in a specific order. Electrons fill the innermost, lowest level energy shells before filling higher energy, outer electron shells. The closer an electron is to the nucleus, the greater its attractive force. Therefore, electrons closer to the nucleus require more energy to escape that shell. Therefore, only electrons in the outer shell are reactive, while interior electron shells are stable and non-reactive. The outermost electron shell, known as the valence shell, fills last under standard conditions, and the electrons in this shell, known as the valence electrons, determine the energetic stability of the atom and its tendency to react to form chemical bonds with other atoms. Similarity in the number of valence electrons explains why elements in the same group share chemical properties.
Figure 6. Modified Bohr model of the atom for atoms in the first three periods. Electrons orbit the nucleus in discrete orbits. Each period represents the number of electron shells an atom of an element has. Electrons are arranged based on the element’s position on the periodic table. Electrons fill the inner shells first as they are lower energy, then add to outer shells.The outer shell, the valence shell houses the valence electrons, which are primarily responsible for an element’s chemical reactivity. The first three shells can hold 2, 8 and 8 electrons, respectively.
Using the Bohr model, the organization of the electron shells can be predicted by the periodic table (fig. 6). The number of possible electrons in each electron shell is equal to the number of cells in the row (or period). In the first shell, there can be up to two electrons, and the second and third shell can hold up to eight each. This is known as the octet rule, which states that atoms are energetically stable when they have a full valence electron shell. Maximizing energetic stability is the foundation of chemical bonding.
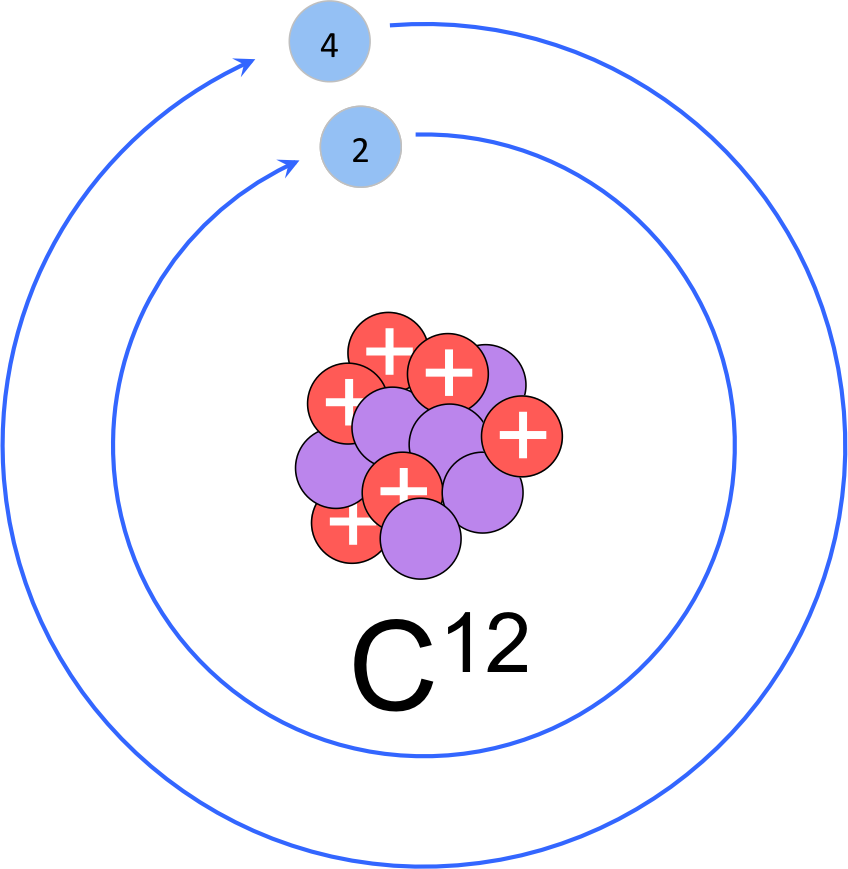
Figure 7. Modified Rutherford-Bohr model of carbon-12. Carbon-12 is the most common isotope of carbon with six protons, six neutrons and 6 electrons (two in the innermost electron shell and four valence electrons.
Let’s construct a modified Bohr model of a carbon atom (fig. 7). As we determined above the nucleus of carbon has six protons and six neutrons. The period (or row) in which an element is in determines the number of electron shells. Since carbon is in the second period it has two electron shells. Of the six electrons, the interior orbital fills first with two electrons and the valence shell contains the remaining four electrons, known as the valence electrons. Determining the number of valence electrons is as simple as counting from left to right in the period. While carbon has four valence electrons, it’s neighbor to the right, nitrogen (element 7) has 5 valence electrons; oxygen has 6 valence electrons; and fluorine has 7 valence electrons. Neon has a completely full valence shell with eight electrons, and is therefore unreactive with other elements. All elements in group 18 are unreactive due to their complete valence shells, and are known as the noble gases. Chlorine is atomic number 17, meaning it has a nucleus with 17 protons. The atomic mass rounds to 35, meaning the most common isotope has 18 neutrons. Chlorine is in the third period; therefore it has 3 electron orbitals with two electrons in the innermost shell, eight in the second shell and seven valence electrons in the outermost shell.
Understanding the positioning of electrons allows us to model chemical interactions. With this model, the repeating patterns of chemical properties found in the periodic table can be explained as a function of the valence electrons. Atoms seek energy stability by interacting with other atoms in attempts to fill their valence shell, either through electron sharing or stealing. While the noble gases are non-reactive, atoms within the first column of the periodic table (i.e. hydrogen, lithium, sodium, etc.) are extremely reactive.
Figure 8. Electron cloud model for the 1s orbital. Rather than the electrons orbiting the nucleus at a discrete radius predicted by the Bohr model, the atomic orbital model predicts electrons are located somewhere within high probability areas (known as orbitals) surrounding the nucleus.
The Atomic Orbital Model
While the Bohr model of the atom is useful in explaining how atoms interact with each other via chemical bonding, it was quickly discovered the concept of electrons circling the nucleus similar to planets did not accurately describe their actual spatial positioning. In 1925, Erwin Schrödinger and Werner Heisenberg proposed a new model of the atom, known as the electron cloud model or the atomic orbital model, which suggested that identifying the exact location of an electron at a specific time is impossible. This model emerged as quantum mechanics discovered electrons have properties of both a wave and a particle, known as the wave-particle duality, indicating electrons can be any distance from the nucleus, however electrons have a much higher probability of being with certain areas.
The innermost shell contains the 1s orbital, which is one spherical orbital of one subshell, and can hold up to two electrons. Essentially, this orbital is exactly the same shapte as the Rutherford-Bohr model; however the precise location of the electron is not predictable. The hydrogen electron configuration is denoted as 1s1, meaning the atom has one electron (indicated by the superscript), in the first s orbital. Helium’s electron configuration is denoted as 1s2, with two electrons in the first s orbital. While the first shell has one orbital (1s) that can hold two electrons, the second electron shell can hold up to 8 electrons, two in a second spherical orbital (2s) and six in three p orbitals (2p). The electron configuration of carbon is denoted as: 1s22s22p2. This indicates that there is full inner electron shell (1s2) with a second shell containing a full, spherical s subshell (2s2 ) and two electrons in separate dumbbell-shaped 2p orbitals (2p2).
For more information on the atomic model, you can visit this website. For our purposes, we will use the Bohr model, as it is a simpler model that adequately describes most basic biological reactions.
Molecules
Molecules are compounds of two or more atoms connected together by one or more chemical bonds. When all electron shells are full, the atom is energetically stable. All elements in group 18, including argon, are known as the noble gases, as they contain complete valence electron shells making them unreactive with other atoms. If an atom does not have a complete valence electron shell, it is energetically unstable, which will interact with neighboring atoms in order to obtain electrons. This reaction forms a chemical bond, increasing the stability of the electron configuration of the atoms within the bond.
Chemical reactions
Electrons of an unstable atom are obtained by either donating, accepting or sharing electrons with neighboring atoms, forming a chemical bond. A chemical reaction is a reorganization of atoms or molecules, known as reactants, producing new atoms or molecules, known as products. For example, water (H2O) can be formed from reactant atoms: two hydrogens and one oxygen. This reaction is expressed by the chemical equation: 2H + O → H2O. The reactants are shown on the left and the products are shown on the right. This equation represents a balanced equation. Matter is not created, nor destroyed. In this reaction, two hydrogen atoms (2H) interact with one oxygen atom (O) to form water (H2O), which has two hydrogen atoms and one oxygen atom. The arrow in between represents the direction of the chemical reactions, which are not always unidirectional. Water can also be split to form hydrogens and oxygen, as well, represented by this equation: H2O → 2H + O. The formation of water is an endothermic reaction, which means that it requires an energy input, In contrast, splitting water releases energy, an exothermic reaction. This is an example of a reversible chemical reaction, which is more accurately written as: 2H + O ⇋ H2O.
Ionic bonds
Figure 9. Ionic bonding in sodium chloride (NaCl). Sodium with one valence electron has a low electronegativity and donates its electron to a neighboring chlorine, an element with high electronegativity. With the loss of an electron sodium has a +1 charge and becomes a cation, and chlorine has a -1 charge and becomes an anion. The differential in these charges forms an strong electromagnetic attraction between the ions, known as an ionic bond. Source.
Certain elements have a tendency to either gain or lose atoms entirely, in order to maximize energetic stability. This occurs when elements have significantly different electronegativities, or the tendency of atoms to attract electrons. Elements with a high electronegativity tend to steal electrons when they come in contact with elements with low electronegativity. Elements that have one or two valence electrons have low electronegativity, which gives them a tendency of donating their electrons to elements of higher electronegativity, which are characterized by a nearly complete valence shell.
Atoms with a different number or protons than electrons are known as ions, which have an electrical charge due to this discrepancy. Ions with a positive are known as cations, which have a positive charge due to the presence of more positively charged protons than negatively charged electrons. Electrons from cations are accepted by certain elements that have a nearly full valence electron shell, known as anions. Anions have more electrons than protons, giving them a negative charge. The difference in the charge between cations and anions holds them together by an electromagnetic force, forming an ionic bond.
Table salt is composed of the molecule, sodium chloride (Na+Cl-). Sodium (Na) only has one electron in its valence shell, and requires less energy to donate its electron than it does to attract seven electrons to fill its valence shell. On the other hand, chlorine (Cl) has a valence shell of seven electrons, and requires less energy to obtain a single electron to fill its valence electron shell than donating all seven of its electrons. This gives chlorine a strong attractive force relative to sodium. When sodium and chlorine interact (fig. 9), an electron is removed from the outer shell of sodium and is transferred into the valence electron shell of chlorine, in a process known as electron transfer. This chemical reaction creates ions, in which sodium becomes a cation (Na+), and has a positive charge due to the differential in protons (p+) and electrons (e-): p++ e- = +11 + (-10) = +1. When chlorine accepts the electron, it becomes a negatively charged anion (Cl-): +17 + (-18) = -1. The difference in the electrical charges in these two ions binds the two ions together in an ionic bond, which relative to covalent bonds are very strong.
Covalent bonds
Figure 10. Covalent bonding of between two hydrogens. Source.
A covalent bond forms when electrons are shared between two atoms. The sharing of electrons occurs between atoms of elements with similar electronegativities. For example, two hydrogen atoms can bind to form a hydrogen molecule, H2 (fig. 10). In this example, each hydrogen atom has a valence shell with a single electron. To complete the valence electron shell, a hydrogen atom can share its electron with another hydrogen atom to complete the valence shells of each atom, forming the energetically stable H2.
Lewis dot structures
Figure 11. Lewis dot structures for boron, carbon and nitrogen.
Predicting molecular arrangements of covalently bonded molecules formed by elements in the first three periods can be simplified by using Lewis dot structures. Since the valence electrons determine molecular bonding in covalent bonds, we represent an atom by writing the elemental symbol and its valence electrons. The simplest way to determine the number of valence electrons an element has is by counting from left to right in the row the atom is located. For example, carbon is element six and housed in the fourth cell in the second period. Therefore it has four valence electrons.
The placement of dots in the Lewis structure assists in predicting bonding patterns. If there are fewer than four valence electrons, each electron will be placed on one of the four sides of an imaginary box surrounding the elemental symbol (fig. 11). These represent unpaired electrons, which are reactive and capable of forming covalent bonds. Carbon (C) is represented by four dots and is capable of forming four covalent bonds; boron (B) is represented by three dots as it only has three valence electrons and can form three covalent bonds. Nitrogen (N) has five valence electrons. The fifth valence electron is paired with an existing valence electron of the element. This pair represents two unreactive electrons; meaning nitrogen is capable of forming three covalent bonds with its unreactive valence electrons. Covalent bonding also occurs between different elements, if they have relatively similar electronegativies.
Figure 12. Lewis and skeletal structures of non-polar molecules: methane and molecular oxygen.
For example, methane (CH4) contains a carbon atom covalently bonded to four hydrogen atoms (fig. 12). Each hydrogen shares an electron the carbon, while the carbon shares a single electron with each hydrogen. When two electrons are shared between atoms, a single bond is formed. In methane, the carbon and the hydrogens are energetically stable as their valence shells are full: eight and two electrons respectively.
A double bond can form when neighboring atoms share four electrons. Molecular oxygen (O2) forms when two oxygen atoms, each with six valence electrons, share four electrons with the adjacent oxygen atom (fig. 12). This fills the valence shell for both atoms making them energetically stable. Ethylene (C2H4) is an organic molecule with both single and double bonds. In this molecule, a double bond forms between the carbons and each carbon forms single bonds with two hydrogens.
Triple bonds are possible when six valence electrons are shared between two atoms. Molecular nitrogen (N2) is an example of a triple bond. Molecules with triple bonds are extremely rare in biological organisms.
Non-polar covalent bonds
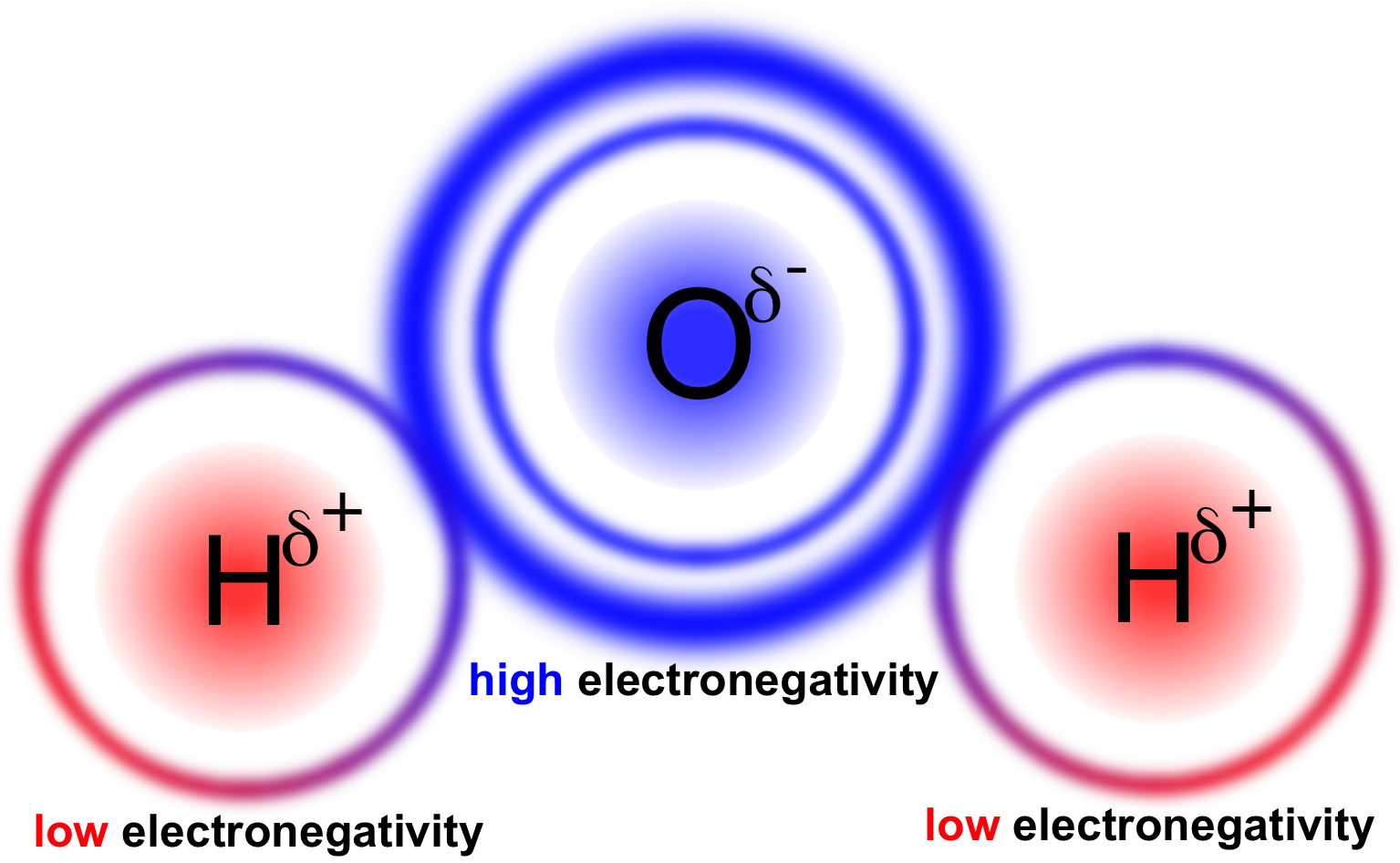
Figure 13. Polarity in water. Oxygen has a higher electronegativity than hydrogen causing it to retain shared electrons more, giving oxygen a partially negative charge and hydrogen a partially positive charge.
There are two types of covalent bonds: polar and non-polar. In a non-polar covalent bond, electrons are shared equally between elements with a very similar electronegativities. Molecular oxygen (O2) is example of a non-polar covalent molecule, as the two atoms are composed of the same element they have an equal attraction of electrons. Methane (CH4) is a non-polar molecule composed of different elements that have similar electronegativities, causing the shared electrons to be shared relatively evenly. While the electronegativities of carbon and hydrogen are not exactly the same, they are similar enough to have a relatively equal distribution of the shared electrons.
Polar covalent bonds
In a polar covalent bond, electrons are unequally shared between atoms of a molecule. This occurs when there is a significant differential in the electronegativity of the atoms. In water (H2O), oxygen has a higher electronegativity than hydrogen, causing the electrons involved in the covalent bond to have a higher affinity for oxygen than hydrogen (fig. 13). This difference in affinity causes the electrons to spend more time orbiting the oxygen, giving oxygen a partially negative (δ-) charge and hydrogen a partially positive (δ+) charge. A polar covalent bond is different from an ionic bond in that the electrons are shared, albeit unequally, between atoms in a polar covalent bond, rather than completely removed and relocated in the case of an ionic bond.
Hydrogen bonding
Figure 14. Hydrogen bonding in water. Hydrogen bonds occur between the partially positive charges of hydrogen and a partially negative oxygen of an adjacent atom, shown here as dashed lines. Media source.
Polar covalent bonds within a molecule is responsible for hydrogen bonding between molecules, such as water (fig. 14). The polarity caused by the partial charges in atoms within a polar covalent bond allow those molecules to form weak attractions with other molecules containing polar covalent bonds. These weak attractions are known as hydrogen bonding, which is an important chemical interaction for living organisms. For example, the two strands of DNA are held together by hydrogen bonding. In the case of DNA, the molecules within a DNA strand are connected by covalent bonds, which are stronger than hydrogen bonds. The difference in bonding strength between covalent and hydrogen bonding allows the strands of DNA to be separated from each other without disrupting the molecules of the DNA strands.
Water: the medium of life
The partial charges from the differential in electronegativity between oxygen and hydrogen gives water many important qualities for living organisms. Water molecules form hydrogen bonds between oxygen and hydrogen atoms of neighboring water molecules. Liquid water is a fluid due to the constant forming and breaking of hydrogen bonds between the partially charged oxygen (δ-) and hydrogens (δ+) of neighboring molecules (fig. 15). The hydrogen bonds between water molecules are broken from the movement of molecules, which is a function of temperature. Hotter molecules move faster. When water reaches its boiling point, the motion of the water molecules is so rapid that hydrogen bonding between water molecules is virtually non-existent, allowing water to change state into a gas, known as water vapor (fig. 15). Ice forms when temperature of the water slows the motion of water to a point where all of the molecules form a lattice-shaped crystalline structure in which all potential hydrogen bonds are formed (fig. 15). Since water molecule is bent, the water molecules in ice are more spread out than in liquid water causing ice to take up more space. This phenomenon is responsible for ice being less dense than liquid water, which is why ice floats.
Figure 15. States of water. Water vapor is gaseous water molecules nearly devoid of hydrogen bonding. Water is liquid due to the constant formaing and breaking of hydrogen bonding (blue dashed lines) among neighboring molecules. Ice forms when all potential hydrogen bonds are formed among neighboring molecules. Due to the water bent structure, ice molecules are more spread out relative to liquid water, causing it to be less dense. Media source.
Figure 16. Water has high heat capacity.. Relative to land, water's high heat of vaporization allows it to absorb more energy causing air temperatures above water to be cooler.
Water has other interesting characteristics. Hydrogen bonding between water molecules gives water an extremely high heat capacity, which is the amount of energy necessary to change the temperature of a substance (fig. 16). Relative to land, water absorbs more heat energy from the sun, while land reflects more heat energy, causing air temperatures directly above water to be cooler. Coastal areas are cooler due to their proximity to cooler air masses above the water, which is created by the water’s high heat capacity relative to the land. The differential in heat capacity in water and land explains why coastal areas experience cooler temperatures in the summer, a phenomenon known as the ocean moderation effect. Water absorbs not just heat, but light as well. If you fly over a beach, the sand appears white and the ocean appears dark blue. Sand reflects back nearly all the visible light waves emitted by the sun causing it to look nearly white; whereas water absorbs nearly all the light except for blue (which is refracted and reflected) making it appear dark blue.
Why do humans sweat? In addition to a high heat capacity, water also has a high heat of vaporization, which is the amount of energy to convert a liquid into a gas. Evaporation occurs at the surface of liquid water when water molecules gain enough energy to break away and become gaseous, a process that rehydrates air masses. Since water requires a substantial amount of energy to evaporate, the area where evaporation is cooled loses heat, a trait that humans utilize to maintain cooler body temperatures as sweat evaporates.
Figure 17. Water as a solvent. Water's partial charges interact with ionic compounds (i.e. NaCl) and partial charges of polar molecules. Water can disassociate atoms in ionic molecules, and the partial charges form a stable structure surround the ions, known as a sphere of hydration.
Water’s polarity provided by its partial charges (δ-, δ+) make it a solvent, or a substance capable of dissolving polar and ionic bonds. In fact, water is the best known solvent, dissolving more substances, known as solutes, than any other liquid. This is one reason that table salt (NaCl), an ionic molecule, dissolves so readily in water. Water’s partial charges bond with polar and ionic molecules, and these molecules become encircled by water in a solution. When water and ionic molecules interact, ionic compounds are disrupted in water and enveloped by a sphere of hydration. The ions react with the partial charges of the water molecules, causing ionic molecules to disassociate, or break apart.
For example, when NaCl is placed in water, NaCl disassociates into the ions, Na+ and Cl- (fig. 17). The Na+ ions are surrounded by the δ- of the oxygens of several water molecules in a sphere of hydration, while the Cl- ions are surrounded by a sphere of hydration connected to the δ+ hydrogens of several water molecules.
Polar molecules (i.e. table sugar) also readily dissolve in water. However, unlike ionic molecules the covalent bonds are not broken in polar molecules. Rather the partial charges of water interact with partial charges of another polar molecule forming hydrogen bonds. Similar to the sphere of hydration in ionic compounds, water forms what is known as a solvent cage around the polar molecules. Non-polar molecules (i.e. oil) do not have partial charges and do not dissolve in water.
Figure 18. Insects can walk on water due its cohesive property.
Insects can walk on water due to surface tension, a product of cohesion, which is the property of like molecules to stick together (fig. 18). Cohesion forms a solid-liquid state at the surface of water due hydrogen bonding, in which water molecules align in a regular pattern. Whereas in the bulk of liquid water, molecules are pulled in every direction producing a randomly assorted pattern. Surface tension is also responsible for the spherical nature of water droplets. For a biological example, water’s cohesive properties allow plants to move water against the force of gravity up their stems. Movement up stems is also affected by water’s strong adhesion properties, which is the ability to bind to other substances. Water binds to the cell walls of plant cells moving up against the force of gravity via capillary action. Adhesion of water also explains why water moves up a glass test tube or graduated cylinder. The molecules of glass have charge which bind to water via hydrogen bonding.
Carbon: the foundation of life
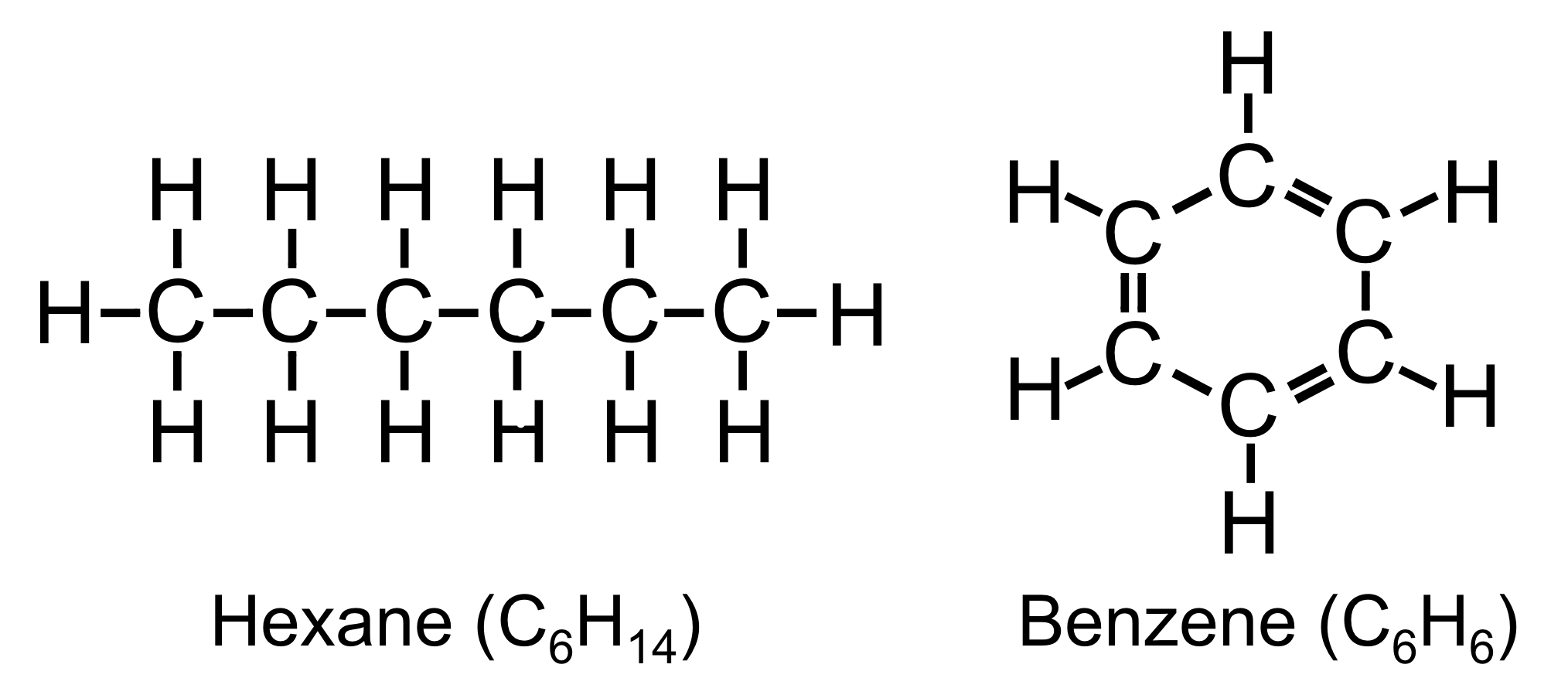
Figure 19. Skeletal structures of hexane and benzene.
As the lightest element with four valence electrons, carbon has the unique ability to form a variety of stable covalent bonds with many elements, including itself. The ability to form four covalent bonds allows carbon, in concert with other elements, to form a large variety of molecules, many of which are quite complex. While carbon can bind with itself to form linear chains, such as hexane (C6H14), carbon can also form ringed molecules. A relatively simple organic (carbon-containing) compound, benzene (C6H6), is an example of ringed organic molecule. To make this molecule energetically stable, each carbon needs to form four covalent bonds, while each hydrogen needs to form one. Each hydrogen binds to one carbon, and the carbons bond to each other with a double bond and a single bond on each side of the carbon, forming a hexagonal structure. Linear and polygonal structures are both common in biological molecules.
Of the nearly 10 million biological molecules discovered, nearly all of them can be placed into four categories: carbohydrates, lipids, proteins and nucleic acids. Simple carbohydrates (i.e. sugars) provide energy to cells via fermentation or cellular respiration. More complex carbohydrates can serve as energy storage (i.e. starch) or structural support (i.e. cellulose and lignin in plants). Lipids are a variable group of molecules that serve many different functions in living organisms, including phospholipids which serves as the foundation for cell membranes, energy storage found in fats and oils, and vehicles for cellular messaging, as is the case with steroids. Proteins serve as the cellular machinery and collectively provide many functions including: speeding up chemical reactions, transportation of materials into and out of the cell, and protection from infections. Nucleic acids include DNA and RNA, which serve as the informational storage and messaging required for the production of the cell’s proteins.
© 2018 Jason S. Walker. All rights reserved.