Much to the irritation of the philosopher, life can be thought of as nothing more than complex series of chemical reactions. To understand the nature of chemical reactions, we first have to be able to determine the structure of the atom. In this series of exercises students will be introduced to basic chemical properties of atoms and molecule, focusing on atoms and bonding most important in biochemistry.
Exercise A: Drawing the Bohr model of atoms
Calculating Subatomic Particles
Atoms have three subatomic particles: protons, neutrons, and electrons. Protons and neutrons are concentrated in the center of the atom, or nucleus, and make up nearly all of the atomic mass. While protons have the same mass as neutrons, protons have a positive (p+) charge, and neutrons (n) are electromagnetically neutral. Elements are defined by number of protons the atom has, while elements can have different numbers of neutrons. Electrons are virtually massless particles orbiting around the nucleus. This attraction is caused by the negative charge of an electron (e-) being pulled toward the positive charge of the proton (p+) . To maintain electromagnetic neutrality, a single atom has exactly the number of protons (p+) as electrons (e-).
So how can we determine the number of protons, neutrons and electrons of an atom? For this we consult the Periodic Table of Elements. Let’s look at Helium. Helium (He) has an atomic number of 2. The atomic number is equal to the number of protons that are present in an atom of helium, and also indicates the number of electrons a single atom of helium will have. The atomic number is always a whole number.
Figure 1. Various ways of representing an atom of the element, helium (He). a) Helium as represented on the periodic table. Helium has an atomic number of 2 (indicating it contains 2 protons and 2 electrons) and an atomic mass of 4. Using the formula, AM = protons + neutrons, to determine that helium has 2 neutrons. b) Helium as represented by the Bohr model of an atom. The protons and neutrons are represented in the middle of the atom, or nucleus. Helium has one electron orbital. You know this because it is in the first row, or period. The first period include H and He, and therefore only two electrons can occupy the first orbital. The outside orbital is known as the valence orbital, and the electrons of the valence orbital are known as valence electrons. Helium has two valence electrons. c) The electron dot diagram of helium. Also known as the Lewis dot diagram, the electron dot diagram is a simplification of the Bohr model, indicating only the number of valence electrons. Helium has two valence electrons, which is represented by a two dots. Since the inner orbital only can only hold two electrons, the valence orbital is said to be filled. This causes an atom of helium to be unreactive.
The atomic mass refers to the sum of the protons and the average number of neutrons of an element. Mathematically:
atomic mass = protons + neutrons
Protons and neutrons each have the same mass, known as an atomic mass unit. We can use atomic mass to determine the number of neutrons. For example, Helium has an atomic mass of 4.00. We can determine the number of protons by plugging in the atomic number (AN=p+) and the atomic mass (rounded) into the equation: 4 = 2 + n. Solving for n, we discover that Helium has 2 neutrons. Please take note that not all atoms have the same number of protons and neutrons (i.e. lithium).
Constructing a Bohr Model of an Atom
Understanding electron configuration is key to understanding chemical reactivity. While we can determine the number of electrons from the atomic number, their configuration needs some additional explanation. Electrons tend radiate around the nucleus at distinct distances, known as orbitals. The structure of the periodic table can help you determine the number of orbitals an element has and how many electrons are in each orbital.
The periodic table is broken into periods (rows) and groups (columns). Each period represents the number of electron orbitals an atom of an element has. We can also determine how those electrons are arranged based on the element’s position on the periodic table. Electrons fill the inner orbitals before adding new orbitals. For example, Lithium (Li) has two orbitals because it is in the second period (second row). Since Lithium’s atomic number is 3, we know that it has 3 electrons. The first two electrons fill the first orbital, and the last orbital (known as the valence orbital) has one electron (known as the valence electron). In fact all elements that are in the first column (Group 1) have one valence electron, but different numbers of orbitals. This gives them similar chemical properties.
Figure 2. Various ways of representing an atom of the element, lithium. a) Lithium as represented on the periodic table. Lithium has an atomic number of 3 (indicating it contains 3 protons and three electrons) and an atomic mass of 6.94. Rounding the atomic mass to 7, you can determine that lithium has 4 neutrons. b) Lithium as represented by the Bohr model of an atom. The protons and neutrons are represented in the middle of the atom, or nucleus. Unlike helium, lithium has two electron orbitals. You know this because it is in the second row, or period. The inner orbitals must fill with electrons before outer orbitals acquire electrons. The number of possible electrons per orbital are equal to the number of elements within a period. The first period have two elements, and therefore only two electrons can occupy the first orbital. Lithium has three electrons. Therefore the first two electrons fill the first orbital and the remaining electron occupies the second orbital. The outside orbital is known as the valence orbital, and the electrons of the valence orbital are known as valence electrons. There are 8 elements in the second period indicating that the second orbital can hold up to 8 electrons. c) The electron dot diagram of lithium. Lithium has one valence electron, which is represented by a single dot.
Exercise B: Ionic Bonds
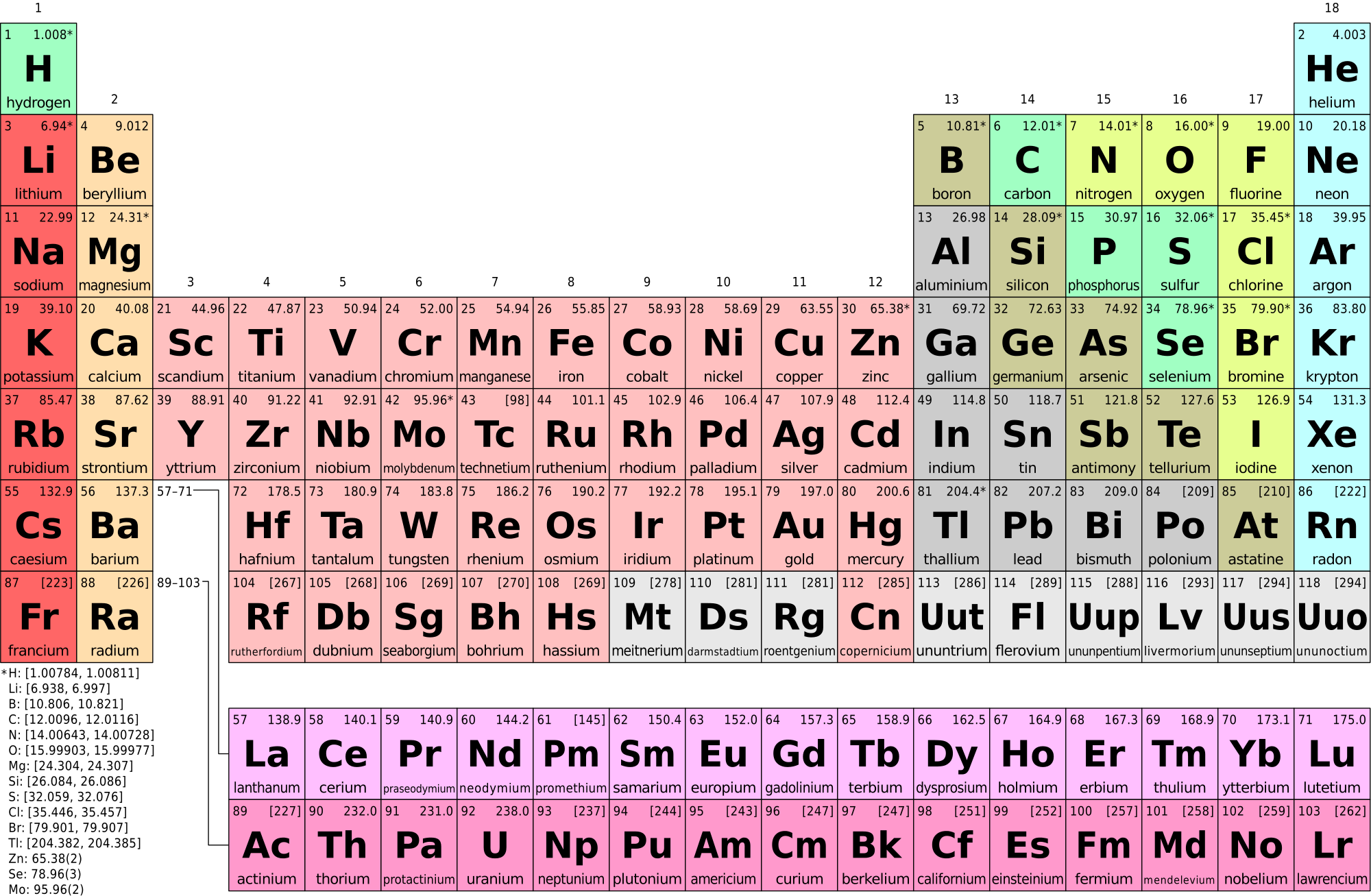
Fig. 3. The Periodic Table of Elements. For this lab, we will only concentrate on the first three rows (or periods). Each period represents the number of electron orbitals an atom of an element has. For example, element 6, carbon (C) is in the second period indicating it has two orbitals. We can also determine how those electrons are arranged based on the element’s position on the periodic table. The number of electrons per orbital is equal to the number of elements within a period. The first period has two elements, indicating the first orbital can have up to two electrons. The second orbital can have up to eight. Electrons fill the inner orbitals before adding new orbitals. To determine the number of valence electrons (electrons in the outer orbital), you can simple count from left to right on the period the element is in. For example, carbon (C) has 6 electrons. It is in the second row, and therefore has two orbitals. Carbon's first two electrons are in the inner orbital and the last four are in the valence electron shell. If you count from left to right in period 2, carbon is the fourth element. This corresponds to the number of valence electrons that carbon has, four. Valence electrons are primarily responsible for an element’s chemical reactivity.
Ionic bonds are extremely strong bonds between atoms with a highly unequal number of valence electrons. Ionic bonds are formed when charged atoms, or ions, are attracted when one gives up one or more of its electrons to the other atom. An ion is an atom or a molecule in which the total number of electrons is not equal to the total number of protons, giving the atom or molecule a net positive or negative electrical charge. Elements that freely give up electrons are known as cations, which have a positive charge due to the loss of an negatively charged electron (-1 x -1 = +1). Cations are elements in Group 1 and Group 2. Elements in these groups have their valence shell nearly complete have a strong negative charge and steal cation’s electrons (+1 x -1 = -1). Such elements are known as anions, and are typically found in Group 16 and Group 17. Ionic bonds occur due to the electrostatic force of attraction between two oppositely charged ions: cations (+) and anions (-).
Table salt is a classic example of an ionic bond. Chemically, salt is known as sodium chloride and has the chemical formula, NaCl. The chemical formula tells us that one atom of sodium (Na) combines with one atom of chlorine (Cl). Let’s see how this bond is formed.
Figure 4. Ionic bond between sodium (Na) and chlorine (Cl) to from sodium chloride, Na+Cl-. The loss of a negatively charged electron (e-) of a sodium atom creates a positively charged sodium cation (Na+), whereas the chlorine becomes a negatively charged anion (Cl-) due to the gain of an electron.
Below is an example of an animated visualization how ionic bonding works in sodium fluoride Na+F-. Like chlorine, fluorine also has one valence electron. So the ionic bond forms similarly to Na+Cl-.
Figure 5. Ionic bonding of NaF. Sodium (Na) gives up one electron to fluorine (F). Since Na lost one negatively charged electron (e-), it has a charge of +1 (-1 x -1 = +1). Fluorine has a charge of -1 since it gained an electron.
Exercsie C: Lewis Dot Diagrams
Chemical reactivity is predominately affected by the number of valence electron atoms have. The electrons of inner orbitals tend to be extremely stable and unreactive. In contrast, the electrons in the outer orbital (the valence orbital), actively react with other atoms. Lewis dot diagrams were developed as a way to simplify the Bohr model of the atom to more efficiently visualize interactions of the valence electrons.
Figure 6. Lewis dot diagram of nitrogen. Nitrogen has five valence electrons. Two valence electrons will pair with each other and be unreactive in a covalent bond. The other three unpaired valence electrons will react with other atoms in a covalent bond.
To construct a Lewis dot diagram, determine the number of valence electron an atom has. For example, nitrogen (N) has five valence electrons. You can easily determine this by locating nitrogen on the periodic table (atomic number 7). In the first three periods of the table, simply count from left to right in the period in which nitrogen is located. Nitrogen is the fifth element in the second period. Therefore, nitrogen has five valence electrons.
For elements in the first three periods (and all elements in groups 1-2 & 13-18), electrons in an orbital tend to pair up when there are more than four valence electrons. In the example of nitrogen, two of the electrons pair up and are unreactive, leaving three electrons to react with neighboring atoms. For the Lewis dot diagram, we diagram this phenomenon by pairing two electrons on one side of the chemical symbol (indicating non-reactivity of those electrons) and placing the other three reactive electrons on the other three sides of the chemical symbol.
Exercsie D: Covalent Bonds
Covalent bonds involves the sharing of electron pairs between atoms, when there is relatively equal attraction due to similar electronegativity between atoms. This relative equality in electromagnetic attraction allows these atoms to share electrons between atoms, bonding them together. Atoms in covalent molecules share enough electrons to complete their valence shell.
Figure 7. Using a Lewis dot diagram to visualize how water (H2O) is formed by covalent bonding. The two molecules of hydrogen have one reactive electron in their valence orbital and each share an electron with a single oxygen atom. Oxygen has six valence electrons, two pairs which are unreactive. The remaining two electrons react with the hydrogen atoms forming single covalent bonds between the oxygen atom and hydrogens.
Figure 8. Skeletal structure of water. Single bonds, in which two electrons are shared between atoms are visualized as a single line between the atoms.
Single bonds
Since hydrogen only has one electron in the first valence shell, it only needs to share one electron from another atom to complete its valence shell. The first shell can only fit two electrons. Elements that covalently bond in periods 2 and 3 (groups 13-16) need to share enough electrons to fill their valence shells, which is eight electrons. The atom of oxygen by itself has 6 valence electrons and needs to share two electrons from neighboring atoms. Each hydrogen atom shares its electron with one of the oxygen’s electrons, pairing up, creating a single covalent bond, typically called a single bond. In a Lewis dot structure, this bond is visualized by the two dots between the H and the O. Oxygen’s four electrons are paired up and are unreactive, which is visualized by the two dots above and below the O. The Lewis dot structure can be simplified into a skeletal structure by highlighting the bonds between the atoms in the molecule, with one line for each two electrons shared of a single bond. The unreactive electrons can be shown in a skeletal structure, but typically they are omitted (yet inferred).
Double bonding
Some molecules can share more than two electrons between atoms. When this happens, double or triple bonds emerge. Oxygen (O2) is an example of a double bond. Each oxygen atom needs to share two of its electrons in order to fill its valence electron. If each atom of oxygen shares its two electrons with another oxygen’s two electrons (four total reactive electrons shared), collectively their valence shells are complete. This is an example of a double bond. The skeletal structure of O2 (Fig. 9) has two lines between the oxygen atoms, representing two pairs of electrons being shared between the two oxygen atoms. A Lewis dot structure of O2 would represent a double bond with four dots between the oxygen atoms, representing the four shared electrons.
Figure 9. Lewis structure and skeletal structure of O2. a) The Lewis structure of O2. An oxygen atom has 6 valence electrons. In O2, two pairs of valence electrons are shared between the two atoms of oxygen, forming a double bond. In a Lewis structure, the double bond is visualized as four dots between the two oxygens. Each oxygen has two pairs of unreactive electrons, shown as dots that are not adjacent between the two atoms. b) The Skeletal structure of O2. O2 is typically visualized as a skeletal structure, with two Os connected by two lines, representing the double bond. Customarily the unreactive electron pairs are not shown.
Exercsie E: Hydrogen bonds
Figure 10. Three dimensional structure of a water molecule. Water is a polar molecule, due to an uneven distribution of electron density. The oxygen in water has a partial negative charge (δ-) due the unequal sharing between the pairs of electrons of oxygen and hydrogen, with oxygen holding the shared electrons more than the hydrogen. In contrast, hydrogen has a partial positive charges (δ+). These partial charges within a water molecule are responsible for hydrogen bonding between water molecules and other molecules, as well.
Water (H2O) is actually a bent molecule. Oxygen has two lone pairs of electrons that do not bond with another atom. Since water is a tetrahedral molecule (think of a styrofoam ball with four toothpicks emerging from it equally distant from each other), the lone electron pairs must be adjacent to each other. When this happens, the lone pairs exert a repulsive force on each other pushing against each other at 121.5˚. Each lone electron pair is also 121.5˚ from its adjacent molecule. This bent nature generates polarity, and bent structure, in the water molecule.
Electronegativity
While a water molecule is formed by covalent bonds, there is unequal sharing of electrons between the oxygen atom and hydrogen atoms. Since oxygen has six valence electrons and hydrogen only has one, oxygen has a stronger pull on the electrons than hydrogen. This pull of an atoms is known as electronegativity.
Oxygen atoms are highly electronegative which attracts electrons to it more strongly than hydrogen, making the region near the oxygen slightly more negative than areas around the two hydrogen atoms. Effectively this makes oxygen partially negative (δ-). In other words, the oxygen atom holds electrons more often than hydrogen. Hydrogen’s low electronegativity is due to its relative inability to hold onto its electrons in the presence of oxygen, gives it a partially positive (δ+) charge. This difference in polarity of water gives its many unique properties. Hydrogen bonding happens between hydrogen and the following highly electronegative elements: oxygen (O), nitrogen (N), and fluorine (F).
Hydrogen bonding in water
In water, hydrogen bonds form between the partially negative (δ-) oxygen atoms of one water molecule with the partially positive (δ+) hydrogen atoms of another water molecule. In liquid water, hydrogen bonding occurs between molecules, but hydrogen bonds are easily broken because they are weak. Therefore, water molecules are continually bonding and detaching. In ice, water molecules bond with neighboring molecules, but they do not detach. Due to the bent structure of the water molecule, the molecular structure of ice forms a lattice network that is more spread out than liquid water.
Figure 11. Hydrogen bonding in liquid water. The electronegativity of a water molecule is responsible for the liquid nature of water. Hydrogen bonds form between adjacent O atoms and H atoms of different H20 molecules. These molecules readily form and detach. Liquid water molecules are randomly assorted and continually attaching and detaching, giving the property of liquid water a fluid state. Liquid water is much denser than ice, because its molecules are more compact (dense) than the crystalline lattice of ice.
Figure 12. Hydrogen bonding in solid water, or ice. Ice is a solid at a molecular level because hydrogen bonds form among all the water molecules forming a lattice structure. This lattice structure (or crystalline structure) is more spread out than liquid water, due to the polarity (and bent shape) of the water molecule. Molecules that are more spread out generate substances that have lower density. This is why ice floats on water.