Chapter 14: Beyond Mendel: the chromosomal theory of inheritance
- Lecture Video: Beyond Mendel
- Lecture Slides: Beyond Mendel
- Study Guide: Beyond Mendel
- Lab: Beyond Mendel
Rediscovery of Mendelian Principles of Inheritance
Figure 1. Gregor Mendel's 1866 publication.
Gregor Mendel presented his work outlining his discrete particulate inheritance model at two scientific meetings in 1865 in Brünn. While those presentations were positively reviewed in local newspapers, the scientific community all but ignored his findings either because they did not understand the findings or just completely discounted them outright. He published his findings in a relatively obscure, scientific journal in 1866 (Fig. 1), but over the next 35 years Mendel’s paper was only cited three times. The strong acceptance of blending inheritance, in which phenotypes of offspring are an intermediate of their parents, was overwhelming causing Mendel’s discoveries to lament in the scientific abyss. It was not until the turn of the 20th century that Mendel’s discoveries were truly appreciated.
In the 1890s, Dutch botanist Hugo de Vries (Fig. 2) began hybridization experiments of different plant varieties including 15 different species. Unbeknownst to him, he was replicating Mendel’s pea experiments. By the turn of the century, the results of his experiments led him to discover what are now known as the principles of Mendelian genetics. During his experimentation de Vries was unaware of Mendel’s work, but that was about to change.
During the same decade, German botanist Carl Correns (Fig. 2) was also experimenting with plant hybridization. In his experiments with hawkweed, he (like de Vries) discovered the underlying principles of Mendel’s experiment without previous knowledge of Mendel. However, as Correns was preparing his manuscript toward the turn of the century, his literature search led him to Mendel’s paper. Realizing that he had rediscovered the same principles he had developed in his own experiment, Correns reconfigured his manuscript as a rediscovery paper used to support and re-introduce Mendel’s study published 35 years earlier.
Shortly before Correns published, de Vries beat him to the punch. In his paper On the law of hybrid disjunction, de Vries provided experimental evidence allowing him to arrive many of the same conclusions Mendel had come to: 1) the presence of dominant and recessive traits, 2) the 3:1 phenotypic ratio found in the F2 generation, as well as 3) independent assortment. However, de Vries did not mention, nor cite, Gregor Mendel’s work. Correns read de Vries’s paper and the day after mailed his manuscript to be published so he could claim dual-priority of the re-discovery of Mendelian genetics. The most significant difference between these two papers was that Correns had read Mendel’s work, and had couched his findings within Mendel’s theoretical framework.
Figure 2. Carl Correns and Hugo de Vries rediscovered Mendelian's principles.
Correns was furious. He realized that de Vries was attempting to lay the foundation for what is now known as genetics, without giving proper credit to the obscure German monk that originally discovered these principles. de Vries claimed his innocence by simply stating that he knew nothing of Mendel’s work. Fair enough, but was it true? Based on an analysis of de Vries writing, it appears that he was truly ignorant of Mendel’s work prior to his first 1900 publication. Prior to 1900, de Vries did not use the terms dominant or recessive, rather he referred to those traits as active or latent. But in his 1900 publication, he referred to them with Mendelian terminology. Coincidence? Maybe. Secondly, de Vries claimed to discover the 3:1 phenotypic ratio in the F2 generation leading to the principle of segregation. However, his actual results were grossly inconsistent within this. Some examples were closer to 2:1 (i.e. 99 hairy to 54 non-hairy), and others like flower color were closer to 4:1. Having ratios range from 2:1 to 4:1 would have not, arguably, led de Vries to independently recognize the principle of segregation. This suggests that de Vries was, most likely, just recently familiar with Mendel’s work at the time of writing his initial 1900 paper, but sought to keep Mendel’s obscure results suppressed, seeking to reap fame as the discoverer of these genetic principles.
Correns not only properly reintroduced the world to Gregor Mendel, he added additional theory. While Mendel was the first to describe hybrids with two letters (i.e. Aa), he notated true-breeding, pure lines from self-pollination with a single letter: A for the dominant trait and a for the recessive. Correns re-interpreted his and Mendel’s results as the presence of a pair of hereditary units, which we now the genotypes (AA, Aa, aa). Correns was the first to make the now famous 9:3:3:1 ratio prediction in the F2 generation, merging the principles of segregation and independent assortment into a single experiment. Correns was also the first to link the concept of segregation with meiosis, a concept not understood in Mendel’s time. Neither de Vries nor Mendel postulated that sex cells (e.g. pollen and eggs) housed one unit of heredity, while vegetative cells contained two.
Many other researchers were working in the field of hybridization at the same time as de Vries and Correns. After reading the 1900 papers reintroducing the world to Mendel, British zoologist William Bateson became one of the leading defenders of the emerging field of genetics, a term which he coined (from the Greek word for ‘decent’). In his book, Mendelian Principles of Inheritance (1902), Bateson translated Mendel’s text for an English audience, and argued that Mendel’s principles would be generalizable to all plants as well as animals. In his writings, Bateson also coined some now ubiquitous terms of Mendelian genetics: alleomorph (subsequently shortened to allele), zygote, homozygote, and heterozygote.
Beyond rediscovery: the origin of the chromosomal theory of inheritance
Figure 3. Walter Sutton discovered the importance of chromosomes in explaining Mendel's principles of segregation and independent assortment.
Following the rediscovery of Mendelian genetics, inheritance research exploded. The next major discovery has become known as the chromosomal theory of inheritance. American biologist Walter Sutton (Fig. 3) began studying the inheritance of grasshoppers at the turn of the century. In 1902, he published a paper arguing that chromosomes occur in matched pairs, with one chromosome from each pair originating from that organism’s parents. In 1903, Sutton expanded his theory to conclude that each pair of chromosomes segregates during the production of sex cells (i.e. sperm or egg cells) via meiosis. In other words, each sex cell (known as gametes) contains a full set of unpaired chromosomes (Fig. 3). Sutton posited that during meiosis, the paired chromosomes aligned randomly and separated. This means that each gamete would contain a random assortment of maternal and paternal chromosomes, explaining Mendel’s principle of independent assortment. During fertilization, the randomized assortment of unpaired maternal and paternal chromosomes of one gamete (i.e. sperm) fuse with the random assortment of another individual’s chromosomes creating a genetically unique offspring.
It is important to note that the chromosomal theory of inheritance is co-credited with the German biologist, Theodor Boveri, who determined that it was necessary for all chromosomes must be present for successful embryonic development of the sea urchin. Furthermore, he discovered that sperm and egg contribute the same number of chromosomes during fertilization. These realizations occurred before Sutton’s 1902-3 publications. However, he did not link those finding to Mendelian principles until a 1904 publication. Interestingly, Boveri was the first to predict that cancerous cells were caused by abnormal chromosomes, a highly provocative hypothesis during his time.
Meiosis explains the principle of segregation
During Mendel’s time, the mechanism of meiosis was not known, but understanding the specifics of meiosis proved instrumental in explaining his results. Mendel’s principle of segregation asserts that an organism has two (assuming diploploidy, 2n) alleles (Mendel called them ‘particles’) for each genotype. A genotype determines a physical characteristic (phenotype, i.e. flower color). Homozygous dominant (e.g. AA) and heterozygous (e.g. Aa) genotypes express a dominant phenotype (e.g. purple flowers); whereas homozygous recessive (e.g. aa) express a recessive phenotype (e.g. white flowers).
Additionally, Mendel suggested that the offspring from a mating will only receive one ‘particle’ (allele) from each parent, and that the probability of getting a specific allele is 50%. In other words, during meiosis, each gamete cell has an equal probability of acquiring an allele of a specific gene. He concluded that the consistency of the phenotypic ratio in the F2 generation (3 dominant: 1 recessive), is only possible if each gamete has an equal probability of acquiring one allele over the second. But how?
A diploid (2n) organism, like pea plants and humans, have two copies of each chromosome known as homologous chromosomes. In humans, one homologous chromosome comes from the mother, the other one from the father. Each chromosome consists of several alleles. Homologous chromosomes have the same sequence of alleles; however the alleles can differ between the homologous chromosomes. Therefore, on one homologous chromosome you can have a dominant allele (e.g. A), while on the other you can have a recessive allele (e.g. a) in a heterozygote; or both homologous chromosomes could have the same allele (e.g. AA or aa) as is the case for a homozygote.
Figure 4. Meiosis explains the principle of segregation. Each gamete resultant from meiosis has an equal probability of acquiring one or another homologous chromosome, for each homologous chromosome pair.
In interphase prior to mitosis, the homologous chromosomes are replicated (Fig. 4). In meiosis I, homologous chromosomes pair up and then split (or segregate) during anaphase I and eventually migrate into two different cells following cytokinesis I. Each cell at the end of meiosis I has either a pair of one identical homologous chromosome or the other.
In meiosis II, the paired identical homologous chromosomes split again, generating the gametes, which have one unpaired homologous chromosome for each chromosome. Gametes have half the number of chromosomes as normal body (somatic) cells. The splitting of the homologous chromosomes explains the 3:1 ratio in the F2 generation of Mendel’s monohybrid cross. Sex cells have a 50% chance of receiving one chromosome or the other for each pair of homologous chromosomes, hence the chromosomal theory of inheritance.
Meiosis explains the principle of independent assortment
Mendel and de Vries both discovered phenotypes they were studying sorted independently. For example, purple-flowered pea plants can have green seeds or yellow seeds, while white flowered plants can also have green or yellow seeds. In other words, flower color was not dependent on seed color. With his 9:3:3:1 phenotypic ratio in the F2 generation, Correns was the first to propose that specific predictions for independent assortment, but he was not aware of the mechanism.
Figure 5. Meiosis explains independent assortment. If alleles are on different chromosomes, there is an equal probability of acquiring any allele combination in dihybrids (i.e. FfSs). This explains the 9:3:3:1 ratio discovered by Correns and Mendel.
The developing understanding of meiosis helped us determine how physical traits were independently assorted. With the emerging understanding of chromosomes, Walter Sutton reasoned that the alleles coding for the different physical traits (or phenotypes) Mendel and Correns were observing were located on different chromosomes (Fig. 5). During meiosis, pair of similar chromosomes, the homologous chromosomes, split during anaphase I and end up in one of two daughter cells at the end of meiosis I. Therefore, each daughter cell at the end of meiosis I contains one of each of the original homologous chromosomes. However, the daughter cells (which eventually become gametes) don’t necessarily have the complete set of original paternal chromosomes, nor the complete set of maternal chromosomes. Gametes end up with a random set of maternal and paternal chromosomes.
Figure 6. Meiosis in humans. Human body cells (or somatic cells) contain 46 chromosomes, which occur in pairs (diploid). During fertilization, 23 chromosomes come from the mother and 23 from the father. Each chromosome from one parent has a similar chromosome from the other, known as homologous chromosomes. During meiosis, the homologous chromosomes in a somatic cell split eventually forming a gamete with half the number of chromosomes (haploid). Each gamete has an equal probability of acquiring either homologous chromosome, known as independent assortment. In this manner, humans can produce 2^23 different possible gametes.
For example, typical human body cells are diploid (2n) composed of 46 chromosomes (notated as 2n=46). A set of 23 chromosomes originated from the father and 23 from the mother (Fig. 6). During fertilization, these sets fuse forming a zygote, the first human body cell. As your body develops, each body cell is composed of these sets of paired homologous chromosomes. When humans reach reproductive maturity, they undergo meiosis to produce gametes, either sperm or egg. During this process, a diploid (2n=46) cell divides the number of chromosomes in half, which then migrate to opposite cells becoming haploid, containing 23 chromosomes (n=23). Each gamete contains one of the original homologous chromosomes for each pair of chromosomes. Independent assortment occurs because the probability of any given gamete receiving either the original maternal or paternal chromosome is 50%. This means that one human can produce 223 (or 8,388,608) different chromosome combinations. This explains why Correns detected independent assortment with his consistent 9:3:3:1 phenotypic ratio in the dihybrid cross of the F2 generation. It just so happened that the phenotypes he was investigating just so happened to be on different chromosomes. He got lucky. Sutton interpreted this result to indicate that the genes coding for Mendel’s and Corren’s different phenotypes were on different chromosomes. And he inferred that the chromosomes were the units sorting independently, not the individual alleles predicted by Mendel. Chromosomes are the unit of inheritance.
Chromosomal sex determinism
Figure 7. Fernandus Payne and Thomas Hunt Morgan.
While Boveri and Sutton hypothesized that Mendel’s principles were related to meiosis, they didn’t have any experimental evidence to support their claim. That evidence came in from the lowly fruit fly, Drosophila melanogaster. Beginning in 1908 American biologist, Thomas Hunt Morgan (Fig. 7), began studying the fruit fly with the hopes of understanding inheritance in animals. At the time, Morgan dismissed both Darwin’s theory of natural selection and Lamarck’s inheritance of acquired characteristics. Rather, he sought to develop experimental evidence to prove de Vries’ mutation theory as the basis of evolution. In this rival theory, de Vries speculated that new species were created nearly instantaneously solely by mutations of the chromosomes. In contrast, Darwin and Lamarck argued that evolution was a gradual process involving selection pressures on a continuum of traits. While they differed in their mechanism, both used the concept of ‘survival of the fittest’ to explain the specifics of their respective theories. In both of these theories, change happens gradually over generations. In contrast, mutation theory predicted that new species arise from mutations instantaneously and at random.
Figure 8. When Morgan crossed a red-eyed male with a white-eyed female, he didn't get one or the other eye color predicted by the principle of dominance. Rather all the male progeny had white eyes and the females had red eyes, the opposite of their parents.
Unlike plants, animals cannot be self-fertilized. He chose the fruit fly for three main reasons. First, they are easy to keep alive...just give them fruit! Second, a fruit fly has an extremely short generation time (about 10 days). So, data from many generations can be accrued in a relatively short amount of time. Third, fruit flies have a tremendous amount of phenotypic variation.
Along with Fernandus Payne (Fig. 7), Morgan began creating mutants of the fruit fly through chemical or radiation exposure. By 1909, heritable mutants began emerging. In 1910, Morgan discovered a white-eyed male mutant, where the all the other fruit flies had red eyes. Flies with the original phenotype are called the wild type. He inferred that the white eye color was a mutation in the chromosomes, and then designed a set of crosses in order to determine how eye color was inherited.
Morgan was shocked to find that the results of the F1 generation in his second cross did not match Mendel’s predictions (Fig. 8). When he mated a white-eyed female with a red-eyed male, he found that all the females of the F1 generation had red eyes, while all the males in the F1 generation had white eyes! But how? Mendelian genetics would have predicted a 3:1 ratio of dominant to recessive phenotypes independent of sex.
During Morgan’s time the understanding of chromosomes was still emerging. As microscopy improved, enhanced visualizations of chromosomes led scientists to discover the sex chromosomes. In 1905, Americans Nettie Stevens and Edmund Wilson independently discovered chromosomal XY sex-determinism. They discovered that males in animals have two homologous chromosomes that determine the animal’s sex: X and Y. The X chromosome looks like an X when it condenses during meiosis, while the Y chromosomes appears more like a V or Y. A male has one of each of these sex chromosomes: XY. Females lack a Y chromosome, having two X chromosomes: XX.
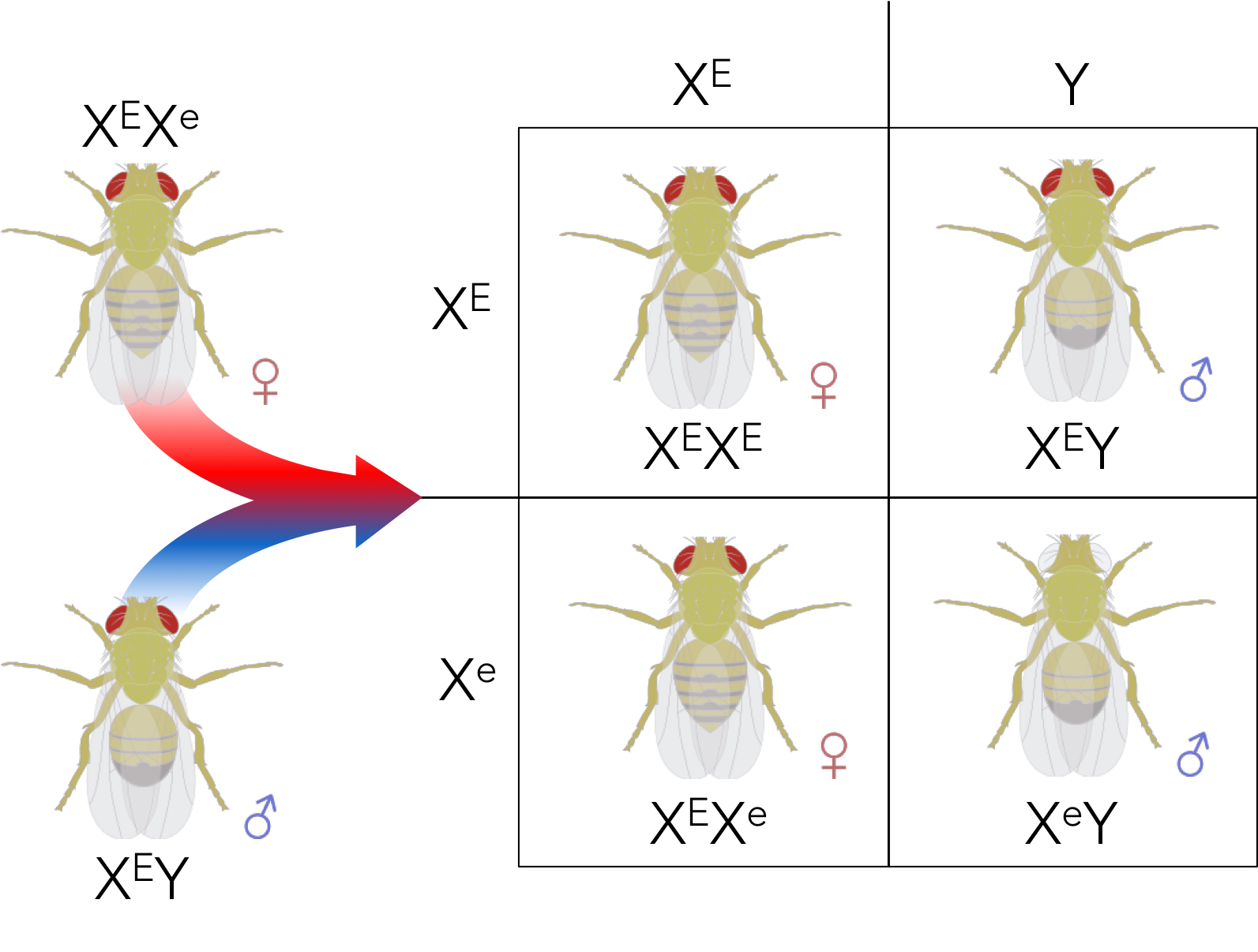
Figure 9. Expected phenotypic ratio of XEXe x XEY, assuming eye color is sex-linked. 2 red-eyed females : 1 red-eyed male : 1 white-eyed male.
The discovery of the X and Y chromosome led Morgan to speculate that the gene controlling eye color of his fruit flies was on the sex chromosomes, specifically the X chromosome. When a gene is located on a sex chromosome, it is said to be sex-linked. Hunt suggested that males expressing the white-eyed phenotype (a recessive trait) only need to have a single allele in order to be expressed, if it is on the X chromosome. The Y chromosome does not have a gene that codes for proteins responsible for eye color. Since X and Y chromosomes are homologous, a white-eyed male would have the genotype: XeY. Since the white-eyed phenotype is a recessive characteristic in flies, he suggested that white eyed female flies must have a recessive allele for eye color on both X chromosomes: XeXe. If this were true, he should be able to predict the phenotypic ratio of controlled crosses, according to Mendel’s principle of segregation.
Figure 10. Expected phenotypic ratio of a cross between a white-eyed female and a red-eyed male. 1 red-eyed female : 1 white-eyed male.
For example, if a heterozygous red-eyed female (XEXe) is crossed with a red-eyed male (XEY), the expected genotypic and phenotypic ratio can be determined using a Punnett square (Fig. 9). The expected genotypic ratio would be 1XEXE: 1XEXe: 1XEY: 1XeY, which gives a phenotypic ratio of 2 red-eyed females: 1 red-eyed male: 1 white-eyed male.
Let’s look at another example (Fig. 10). Construct a cross between a white-eyed female and a red-eyed male. Notice you weren’t given the genotypes, but with this information we know what they are. Since white eyes is a recessive phenotype, we know the genotype is XeXe. Furthermore, since males only have one X chromosome, whatever allele is on that chromosome is expressed. Therefore, his genotype must be XEY. If you construct a Punnett square for this cross (XeXe x XEY), the expected genotypic ratio of 1XEXe: 1 XeY, represented by 1 red-eyed female to 1 white-eyed male.
Gene linkage and independent assortment
With his investigation of eye-color and its relationship to the sex of the fruit flies, Morgan assembled evidence that alleles are attached to specific chromosomes. Morgan found that his expected phenotypes in the F1 supported the hypothesis that genes for eye-color were linked to the X chromosome. This finding had implications for how genes assort during meiosis. Several genes are linked to a single chromosome, and chromosomes are the ‘particles’ separated during meiosis, not the individual alleles suggested by Mendel.
Therefore, Hunt predicted not all alleles should independently assorted as predicted by Mendel. Different genes on the same chromosome should be linked. In other words, genes on the same chromosome should be dependently assorted (originally rejected by the 9:3:3:1 ratio of Correns and Mendel’s dihybrid crosses), whereas genes on separate chromosomes should be independently assorted (as predicted by Mendel). Linked genes violate independent assortment.
Figure 11. Possible genotype and phenotype combinations of eye and body color.
In his experimentation, Morgan discovered another X-linked gene which affects fruit fly body color (Fig. 11) with grey bodies as a dominant phenotype (expressed as XB) and yellow bodies as the recessive phenotype (expressed as Xb). For females, phenotypic expression follows Mendel’s principle of dominance, where homozygous dominant and heterozygous genotypes express the dominant phenotypes: grey body and red eyes. Recessive phenotypes (yellow body and white eyes) are expressed by two recessive alleles: XeXe and XbXb, respectively. On the other hand, males only have one of these alleles as they only have a single X chromosome. Whichever allele is present is expressed: XEY (red eyes), XeY (white eyes), XBY (grey body), and XbY (yellow body).
Since both of the alleles are on the same chromosome (X), we notate the genotypes accordingly. For example, a female with a yellow body (recessive) and white eyes (recessive) would be expressed as XbeXbe, while a male with red eyes (dominant) and a grey body (dominant) is expressed as XBEY.
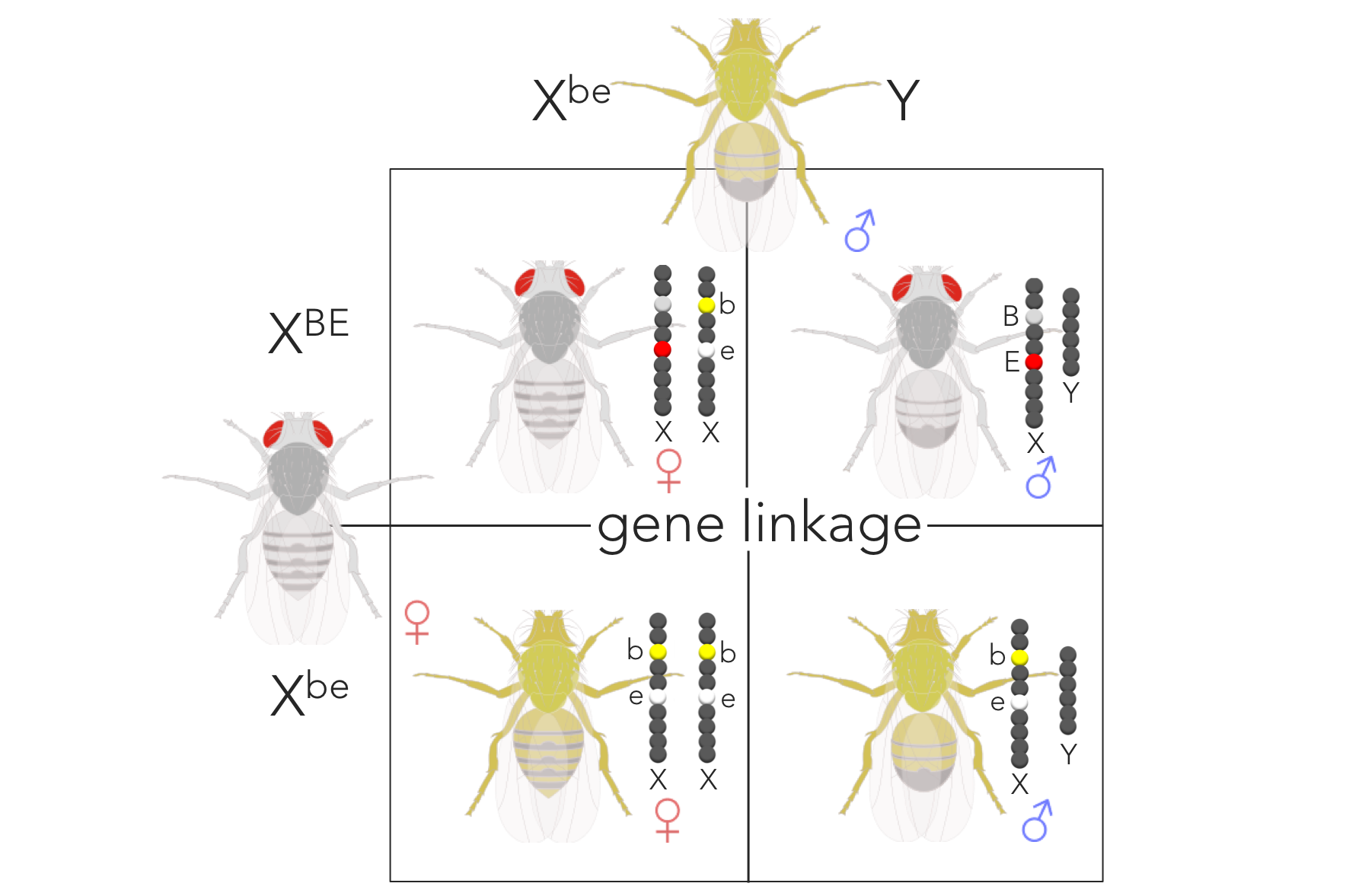
Figure 12. Expected phenotypic ratio of XBEXbe x XbeY supporting gene linkage. Removing sex from the ratio, the expected phenotypic ratio supporting gene linkage is 1 red/grey : 1 white/yellow.
Morgan expected the principle of independent assortment (predicted by Mendel) to be rejected considering based on his belief that alleles for both of these traits are attached to the same chromosome. He conducted a cross of dihybrid females (XBEXbe; grey bodies and red eyes) with recessive males for both traits (XbeY; yellow bodies and white eyes). If we assume gene linkage, the B and E alleles of XBE will stay connected together during meiosis and fertilization. Likewise, the b and e alleles of Xbe will remain linked. By completing a Punnett square (Fig. 12) assuming gene linkage (or dependent assortment), Morgan predicted a genotypic ratio of 1 XBEXbe: 1 XbeXbe: 1 XBEY: 1 XbeY (or a phenotypic ratio of 1 grey/red ♀: 1 yellow/white ♀: 1 grey/red ♂: 1 yellow/white ♂).
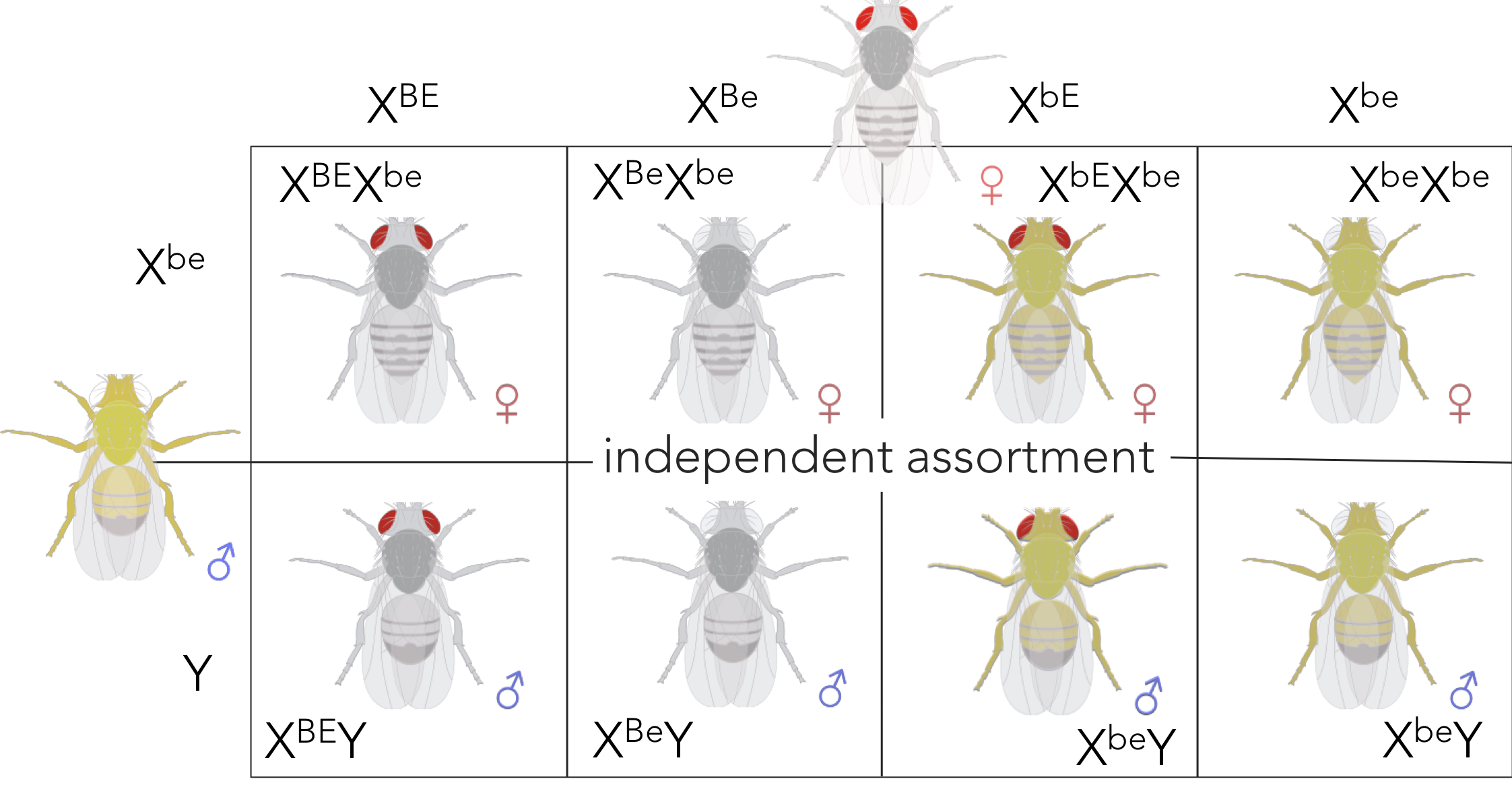
Figure 13. Expected phenotypic ratio of XBEXbe x XbeY supporting independent assortment. Removing sex from the ratio, the expected phenotypic ratio supporting independent assortment is 1 red/grey : 1 white/grey : 1 red/yellow : 1 white yellow.
If you remove the sex of the fly from the equation, he expected an even distribution (1:1) of grey-bodied, red-eyed individuals to yellow-bodied, white-eyed individuals. Gene linkage predicts no grey-bodied, white eyed individuals nor yellow-bodied, red-eyed individuals would be present from this cross (XBEXbe x XbeY). Alternatively, if the alleles for body color and eye color were independently assorted as predicted by Mendel and Correns, he would predict (Fig. 13) an even distribution (1:1:1:1) of the phenotype combinations: 1 grey/red (XBEXbe and XBEY): 1 grey/white (XBeXbe and XBeY): 1 yellow/red (XbEXbe and XbEY): 1 yellow/white (XbeXbe and XbeY).
Discovery of recombination
Results of his F2 generation (XBEXbe x XbeY) did not support either of these alternative hypotheses. In his cross, he acquired: 1159 grey-bodied, red-eyed flies; 1017 yellow-bodied, white-eyed flied; 17 grey-bodied, white-eyed flies; and 12 yellow-bodied, red-eyed flies. While the numbers are closer to the 1 grey/red: 1 yellow/white prediction of gene linkage and dependent assortment, that hypothesis predicted no grey/white or yellow/red combinations. While independent assortment did include those combinations, it predicted an even distribution of each combination. The truth was somewhere in between; but what was the mechanism?
Figure 14. Results from Morgan's cross XBEXbe x XbeY. Morgan's results didn't support either alternative hypothesis leading him to conclude that chromosomes typically segregate in whole, supporting gene linkage, but occasionally homologous chromosomes are recombined in a process known as crossing over, creating recombinant chromosomes. w
Again, the inner working of meiosis is responsible for Morgan’s results. He suggested that alleles that are on same chromosome remain linked during meiosis, but not always. Sometimes alleles on the same chromosomes don’t always stay together. Morgan proposed that during meiosis paired homologous chromosomes can swap segments of chromosomes between them. He referred to these recombined chromosomes (part paternal-part maternal) as recombinant chromosomes.
Further work by Morgan and colleagues supported this hypothesis, and they found that homologous chromosomes do, in fact, recombine during prophase I of meiosis. Morgan termed this process as crossing over, and it involves the physical exchange of segments between homologous chromosomes. He concluded that genes connected to the same chromosome typically remain together supporting dependent assortment, but occasionally there is an exchange of segments between maternal and paternal homologous chromosomes. This explains his result from the cross (XBEXbe x XbeY) resulting in the F2 generation, which is predominantly a 1 dominant/dominant : 1 recessive/recessive ratio with a rare emergence of dominant and recessive combinations in a single individual.
Gregor Mendel was lucky. It turns out the phenotypes he was observing just so happened to be on separate chromosomes. This allowed him to piece together a very simple model of inheritance, the particulate inheritance hypothesis which include the principle of independent assortment. Had his some his traits been on the same chromosome while others were on different chromosomes, he may have not been able to generate a working hypothesis to explain his results. Further research has revealed many other violations of Mendel’s principles.
Incomplete Dominance
Figure 15. Incomplete dominance.
In the early 1900s Carl Correns contributed to the reemergence of Mendelian principles of inheritance through extensive experimentation on a number of organisms. One plant he analyzed was the four o’clock, which have either red, pink or white flowers. After he self-pollinated the red and white flowers, the resultant plants were pure lines, represented by the P generation. According to the principle of dominance, he expected the flowers of the F1 generation to either be all red or all white. To his surprise, all the hybrids of the F1 generation were pink. This result violated Mendel’s principle of dominance and supported blending inheritance. Following the cross of the hybrids, the plants that emerged in the F2 generation did not express the 3:1 ratio that Mendelian genetics predicted. Rather, the phenotypic ratio was 1 red: 2 pink: 1 white flowers. He had discovered incomplete dominance. In this case, both alleles are expressed, not one dominates over the other. In the case of a heterozygote, the flower color is mixture of the phenotypic characteristics of the homozygotes.
Codominance
One of the underlying mechanisms Mendel utilized to explain his results was that each trait had one of two possibilities (i.e. white or purple flowers). In modern terminology, we would say that each genotype is composed of one of two alleles (in homozygotes) or both alleles (in heterozygotes). Investigations into human blood types illustrated some phenotypes are expressed by more than two possibilities. In 1900-01, Austrian Karl Landsteiner discovered that humans have one of three blood types (A, B or O). He discovered that blood transfusions by people of the same blood type did not lead to the destruction of blood cells of the recipient, which became instrumental tool in the medical profession.
Figure 16. Codominance in human blood type.
Different alleles emerge via mutations during DNA replication, and they occur randomly. Therefore, it is possible for a gene to have more than two alleles, and some do. Human blood type is a well-known example. A gene known as I is responsible for the production of a specific polysaccharide attached to a glycoprotein found in the cellular membrane of red blood cells. The recessive allele i codes for a base polysaccharide. The alleles IA and IB code for the same base polysaccharide plus an additional sugar added to the end. IA codes for one sugar, while IB codes for a different sugar. Three blood type genotypes possible: IAIA, IAIB, IBIB, IAi, IBi, and ii. Similar to Mendelian genetics, i is recessive to both IA and IB. Genotypes IAi and IBi result in the type A blood and type B blood, respectively. Where the recessive phenotype is type O blood: ii. But what happens when both IA and IB alleles are present in a genotype? As it turns out, both alleles are activated, and blood cells with the genotype IAIB produce both type A and type B glycoproteins. Neither dominates over the other. This double expression is known as codominance.
Polygenic inheritance
Figure 17. Human skin color is expressed on a continuum as a result of polygenic inheritance. In lower latitudes, selection pressures promote darker skin to protect from cellular damage from UV exposure. Lighter skin promotes higher vitamin D production, essential in the more northern latitudes.
When we think about inheritance in our own species, the principles of genetics discovered by Mendel are difficult to apply. Some of the most obvious phenotypes humans can detect seem to exist on a continuum: such as height, shoe size and flexibility. Human skin color is a classic example. Ranging from dark brown to the lightest hues, skin color is determined by the presence of many substances, but most importantly the pigment melanin. The ancestors of all human originated near equatorial Africa about 1.2 million years ago. Being devoid of thick body hair, those people were assaulted by the sun’s ultraviolet rays. Too much exposure to UV rays is a main determinant for skin cancer. Natural selection favored humans that had a built-in mechanism for reflecting UV rays: melanin. As humans migrated northward, they were dark-skinned (as revealed by recent DNA analyses of ‘Cheddarman’ in southern Britain). However, humans generate vitamin D from UV rays. The less melanin present in the skin, the more essential vitamin D could be generated. Hence there was a selection pressure for lighter skin tone in more northern latitudes. As it turns out, human skin color is not discrete (either/or) as predicted by Mendelian genetics. It is continuous. While the research is incomplete, we now know that skin color is determined by at least seven (likely more) different genes, or polygenic inheritance.
Mendel and the rediscovery of his principles laid the foundation for modern genetic theory. But when we look closer at inheritance, every principle or law that was discovered in those early days has an exception. And those violations of genetic principles have paved the way for additional insight to the inner working of the cell.
© Jason Walker. 2018.