Photosynthesis
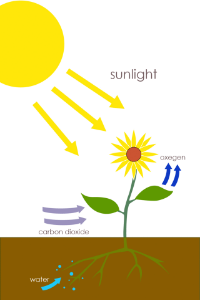
Photosynthesis is the process by which plants and other organisms (i.e. cyanobacteria and algae) convert light energy from the sun into chemical energy. In the process of photosynthesis, carbon dioxide (CO2) and water (H2O) undergo a series of chemical reactions initiated by light energy to produce glucose (C6H12O6) and oxygen gas (O2).
As sunlight shines down on plants, water is absorbed by the root system of the plant. Water is carried up by an internal plumbing system, known as the vascular tissue, up to the photosynthetic tissue (i.e. the leaves).
In the leaves, water brought up from the vascular tissue absorbs into the photosynthetic leaf cells via simple or facilitated diffusion. Carbon dioxide (a gas) diffuses into the leaf directly through specialized mouth-shaped cells, known as guard cells. The holes made by guard cells are called stomata. Carbon dioxide and water go through a series of chemical reactions in the chloroplasts of plants to produce glucose with oxygen as a byproduct.
In the leaf of the plant, there are several different tissues. The upper and lower most tissues are composed of small, boxed-shaped cells known as the epidermis. These cells excrete a waxy substance on the outside of the epidermis, known as a cuticle. The cuticle’s function is to prevent water loss in plants. Cuticles are so effective at preventing water loss, plants had to develop a mechanism for getting carbon dioxide gas into the leaf. Guard cells are able to open and close and are responsible for regulating the plant’s CO2 and H2O levels.
Inside the leaf are two other photosynthetic tissues. Just below the upper epidermis is a layer of tightly packed photosynthetic cells that undergo the majority of photosynthesis in plants, the palisade mesophyll. Directly below this layer are photosynthetic cells that are much more spread out, known as the spongy mesophyll. When the guard cells close to conserve water, this layer serves as a CO2 reservoir, which allows photosynthesis to continue even in a closed system. That is, until all of the CO2 is fixed.
Water is absorbed by the roots of the plant and travels up the vascular system by the tissue known as xylem. Water enters the leaf and absorbs into the photosynthetic cells by osmosis, combining with CO2 to produce glucose and oxygen. Inside the cell, water can be stored in the vacuole.Excess oxygen not used during cellular respiration diffuses to the outside environment via diffusion. Glucose is either utilized by the cell directly, or is shuttled to the vascular tissue that transports glucose to other cells incapable of photosynthesis (i.e. roots) in a vascular tissue known as phloem.
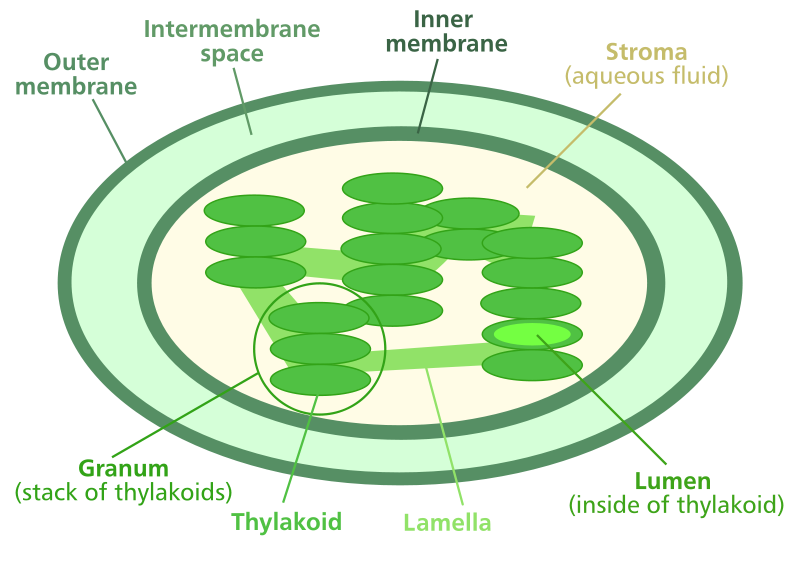
The anatomy of a chloroplast
Comprehending chloroplasts’ internal anatomy will help your understanding of the specific chemical pathways of photosynthesis: the light reactions followed by the Calvin Cycle.
Chloroplasts are ellipsoid organelles with a multiple (2-4) membranes (depending on the organism). Inside the chloroplast are pancake-like structures, known as thylakoids. At the membrane of the thylakoids, the light reactions of photosynthesis occur. Thylakoids tend to form stacks, known as grana. The products of the light reactions travel into the liquid area inside the chloroplasts, known as the stroma. Once those products reach the stroma, they undergo the Calvin Cycle.
Light reactions
When light energy strikes the membrane of the thylakoids, the molecule chlorophyll becomes excited. When this happens, chlorophyll donates electrons in a chemical chain reaction, known as the electron transport chain. It is at this step that chlorophyll has converted light energy into chemical energy. This energy is used to split a water (H2O) molecule into oxygen gas (O2) and a proton (H+). A proton is a regular hydrogen molecule with one proton and no electrons. Oxygen gas is a byproduct and is exported outside of the chloroplast via diffusion. This proton (H+) combines with the molecule NADP+ (nicotinamide adenine dinucleotide phosphate) forming NADPH. NADPH is shuttled out of the thylakoid and gives up its H+ in the Calvin Cycle. The chemical splitting of water actually releases kinetic energy of its own which is used to synthesize ATP (adenosine triphosphate) from ADP and P, in a process known as photophosphorylation. These ATPs are used to initiate the Calvin Cycle.
Calvin Cycle
The three products of the light reactions are NADPH, ATP, and O2. Oxygen is a waste product and leaves the chloroplast (and eventually the plant through the stomata) via diffusion. NADPH and ATP leave the thylakoid, entering into the stroma (the liquid interior of the chloroplast) and enter the Calvin cycle. The Calvin cycle is a very complicated biochemical pathway whose details are beyond the scope of this primer. It is in the Calvin cycle that gaseous carbon dioxide becomes “fixed” into a usable carbon-containing molecule. ATP from photophosphorylation is used to fuel the Calvin cycle. The NADPH also enters into the Calvin cycle, donating its hydrogen (H+). That hydrogen is combined with the carbon and oxygen from CO2 to form the desired product: glucose (C6H12O6).
Lab: Investigating Photosynthesis
Introduction
You will be using a leaf disk assay to investigate photosynthesis. You will use a hole punch to make leaf disks and put these leaf disks in a sodium bicarbonate (baking soda) solution. In water, the bicarbonate solution is a carbon source that the plant can use to undergo photosynthesis (akin to CO2 in air). Initially when you submerge the leaf dish in the bicarbonate solution, the air spaces in the leaf should fill with the bicarbonate solution causing the leaf disks to sink. As photosynthesis proceeds, oxygen gas will be produced and build up in the leaf. As this happens the density of the leaf should become lower than water and the leaf disks should begin to rise. Since plants also use oxygen gas during cellular respiration, this assay is only a relative measurement of photosynthesis. The rate of photosynthesis can be measured by how quickly the leaf disks rise. The faster they rise, the higher the net rate of photosynthesis.
Materials
- Sodium bicarbonate (Baking Soda)
- Liquid soap
- Plastic syringe (10cc or larger) – remove any needle
- Leaf (spinach or ivy are known to work very well
- Hole punch
- Clear plastic cups
- Timer
- Light source
Protocol (Experimental Group)
- Prepare 0.2% bicarbonate solution. Add 1/8 of a teaspoon of sodium bicarbonate to 300ml of water. Stir until the sodium bicarbonate is completely dissolved. Add 1 drop of liquid soap to the solution and mix. This allows the solution to be absorbed by the leaf. If you acquire suds, you will need to add more bicarbonate solution until the suds disappear.
- From your plant material, cut out 10 leaf disks with a hole punch. Be careful to avoid major plant veins.
- Remove the plunger from your syringe.
- Place 10 leaf disks in plunger.
- Place plunger back in the syringe and depress it, being extremely careful not to apply any pressure to the leaf disks. If you crush the leaf disks, the experiment will not work. If this happens, simply cut out more leaf disks.
- Place the syringe in the bicarbonate solution, and draw a small amount (2-3cc) of bicarbonate solution. Tap the syringe to suspend the leaf disks.
- While holding your finger over the opening of the syringe (make sure you removed the needle), draw the plunger back in order to create a vacuum. Hold the vacuum for 10 seconds and swirl the leaf disks to suspend them in the solution.
- After 10 seconds, remove your finger releasing the vacuum. This will allow the sodium bicarbonate solution to infiltrate the air spaces in the leaf. Once this occurs, the leaves will sink. You will likely need to repeat this procedure several times to get the leaf disks to sink. If you can not get your leaf disks to sink after three evacuations, add one more drop of soap to your bicarbonate solution and repeat the procedure. Continue adding soap one drop at a time and repeating the procedure until the leaf disks sink.
- Carefully remove the plunger from your syringe. Pour the contents (leaf disks and bicarbonate solution) in to a transparent glass. Add more bicarbonate solution to the cup to a depth of 3cm.
Protocol (Control Group)
- Repeat the procedure for the Experimental Group, substituting water for the bicarbonate solution.