Chapter: The Cell Membrane
A lipid is a group of compounds that have one thing in common, at least part of the molecule they have little to no affinity to water, due to the presence of a fatty acid chain. They have a varied chemical composition among the different lipids, but this they do have in common. They are hydrophobic. This hydrophobia is due to the nonpolar nature of the carbon to hydrogen bonds that exist on the ends of fatty acids chains.
There are three groups of lipids: fats, phospholipids, and steroids.
Fats
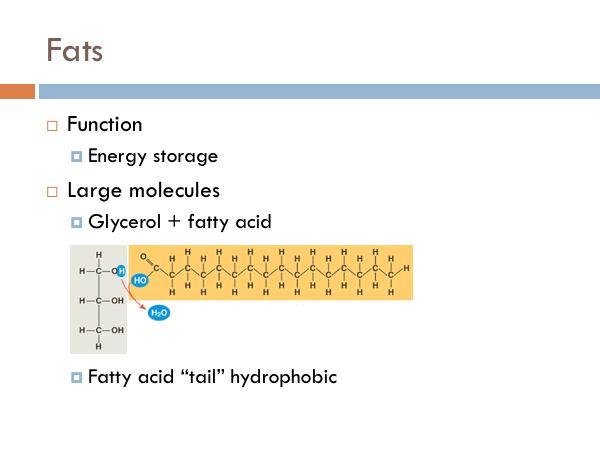
Fats function as energy storage. A fat is defined as a glycerol molecule attached to a fatty acid chain. The fatty acid is a non-polar chain of carbons attached by single bonds to several hydrogens. Those bonds can be broken up in order to go through ATP synthesis. ATP is how all organisms on Earth use chemical energy to do physical work inside the cell. Of the fats, the fatty acid tail is the part that is hydrophobic. If it comes into contact with water, the fatty acid tails turn away from it.
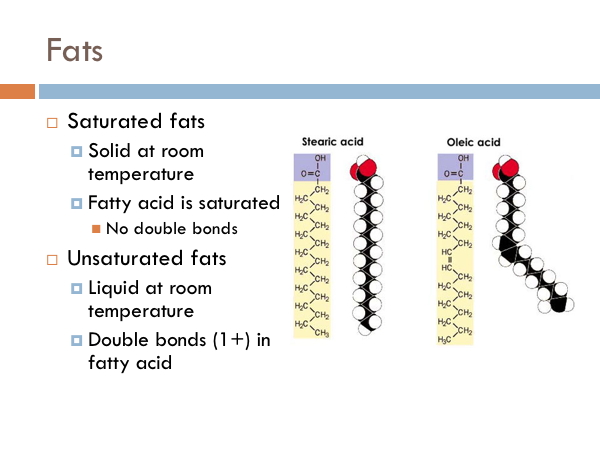
Saturated fats are solid at room temperature. Butter is a great example of a saturated fat. And the reason it is called a saturated fat is because there are no double bonds within the fatty acid tail. Unsaturated fats are liquid at room temperature. Oils are very typically saturated fats. They differ from saturated fats in that they have at least one double bonds. The reason that saturated fats are solid at room temperature is that the molecules are able to pack much closer together. Think of a package of dry spaghetti noodles. The reason that saturated fats are liquids at room temperature is that they can’t fill nearly as much space with the same number of molecules. Think of a bag of dry macaroni noodles. There is a lot of space in there, compared to the spaghetti bag.
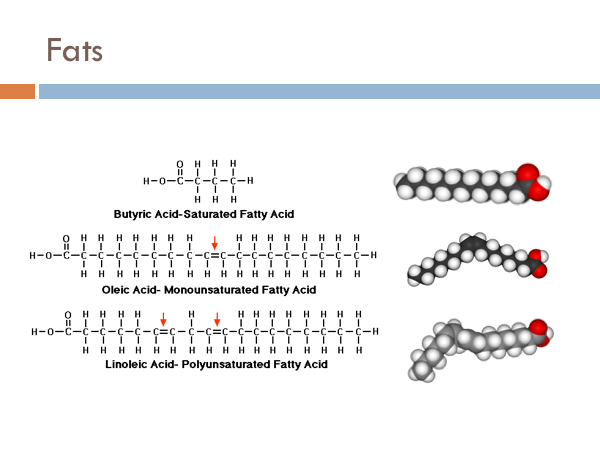
Saturated fats have a glycerol with a fatty chain completely absent of double bonds. Monounsaturated fats have one double bond in its fatty acid chain. Polyunsaturated fats have more than one double bond in the fatty acid chain. Saturated fats are considered bad fats whereas monounsaturated fats and polyunsaturated fats are considered good fats. And you might be wondering why. As it turns out, even though bond strength is higher at double bonds, they are also sites for chemical reactions to take place. Your body has to work a whole lot harder to break apart the saturated fatty acid chains than it does to break apart the unsaturated ones. Once those bonds are broken, they can be used by the body to produce ATP, in order to do the work of the cell. Another reason that Saturated fats are “bad” is that they can break off from the glycerol and reform to produce cholesterol. And cholesterol is another lipid, which are even harder to break down.
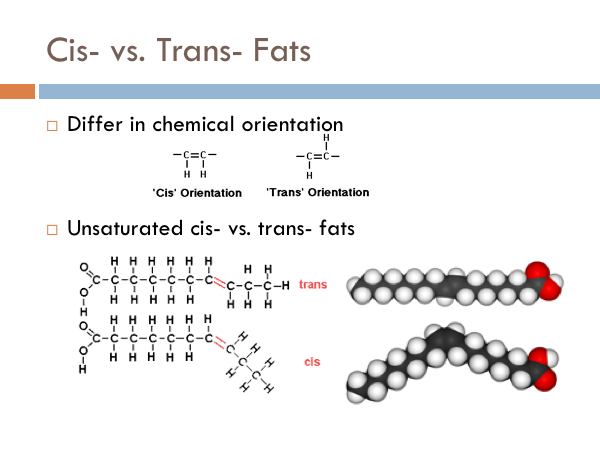
Cis vs. Trans Fats
You have probably heard that trans-fats are bad. But what are they. Chemicals can have the same molecular formula but two different orientations. The two fats pictured to the right have 10 Carbons, 2 Oxygen, and 18 Hydrogens. However, you can see that they look different. This is due to orientation. The one that is bent is said to have a “cis” orientation. It is where the 2 Hydrogen on are on the same side of the double bond. And since like charges repel, the hydrogens repel each other and produce a kink. Trans fats have fatty acids chains that have a hydrogen on opposite sides of the double bond, evening out the charge, leading to a much straighter fatty acid chain. So why does it matter. The kink in the cis fatty acid allows a protein to attach to it causing the fatty acid to get broken down with a lot less energy. The trans fatty acid gets broken down similar to a saturated fat, but once the enzyme reaches the double bond, it requires a lot of energy to break that specific bond.
Phospholipids
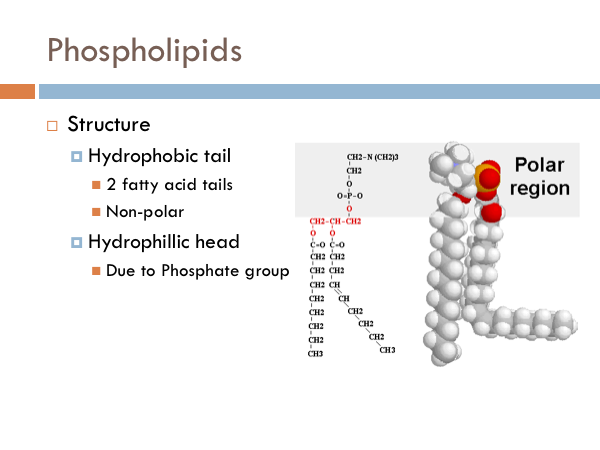
Phospholipids are a group of lipids which are composed of two fatty acids tails combined with a phosphate group. One of those fatty acid tails has a double bond, causing a kink in it. These fatty acids are hydrophobic, they are repelled by water. In contrast, the other part of a phospholipid is hydrophilic. It loves water. It turns toward it. The reason it is attracted to water is that due to its phosphate group. That is the phosphorous atom attached to all the oxygens.
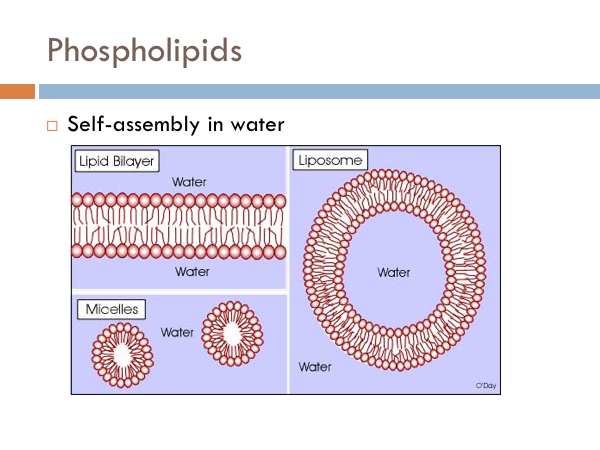
Phospholipids have one side that is attracted to water, and the other is repelled by water. So what happens when you put it in water. There are two possibilities. The simplest structure is where all of the hydrophilic heads face the water and all the tails face away from it, touching tail to tail. This is called a micelle. It reminds of a group of elephants circling up to protect their young ones from predators. But if water gets into the center of that structure, the fatty acid tails turn away from it, while the polar regions turn toward it, creating a lipid bilayer and a structure known as a liposome.
Steroids
Steroids are also hydrophobic. They are defined by a carbon skeleton made up of 4 Carbon rings combined together. On the end of one of those rings is a fatty acid chain, which makes it hydrophobic. Cholesterol is one of the most basic steroids that humans have. It is the precursor for most other steroids that the body produces and uses. Some examples of other steroids are estrogen and testosterone. Steroids are important chemical messages that communicate instructions to the internal cellular machinery.
Cell membrane
A cell membrane is a lipid bilayer embedded with proteins. Just like there were reporters embedded when the troops surged into Iraq. There are proteins embedded in a field of phospholipids. Cell membranes are selectively permeable. They let some things in with no problem. They let other things in with permission, and other things can’t ever get in. It is kind of like a prison gate in that respect. Air can come and go; prison guards and visitors can come and go under specific conditions. But those prisoners are stuck. Therefore, a prison is selectively permeable.
Phospholipid bilayer
Remember phospholipids have hydrophilic heads and hydrophobic tails. When they are in water, the hydrophilic heads turn toward the water and the hydrophobic tails turn away from it. This causes a bilayer (two layers) with water on both sides. This is the basic structure of the cell membrane
Permeability
Cellular permeability is how easily it is to get across the cell membrane. There two basic types. First, there is passive diffusion. This happens when a chemical requires no energy to move across the membrane. Those molecules move from high concentration to low concentration. Active diffusion is the opposite. It requires energy to cross the cell membrane. Specifically, it requires ATP and molecule move from high concentration to low concentration.
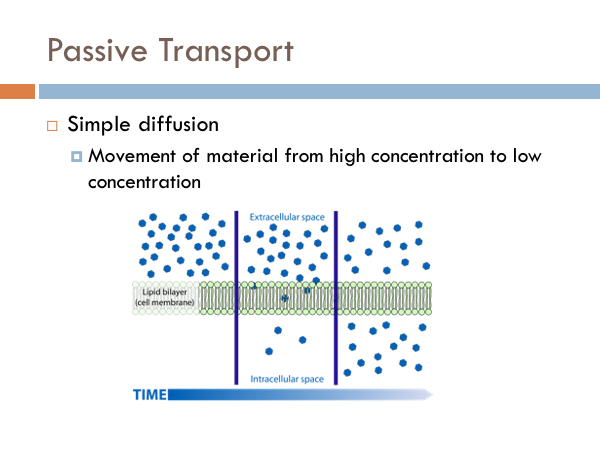
Passive Transport
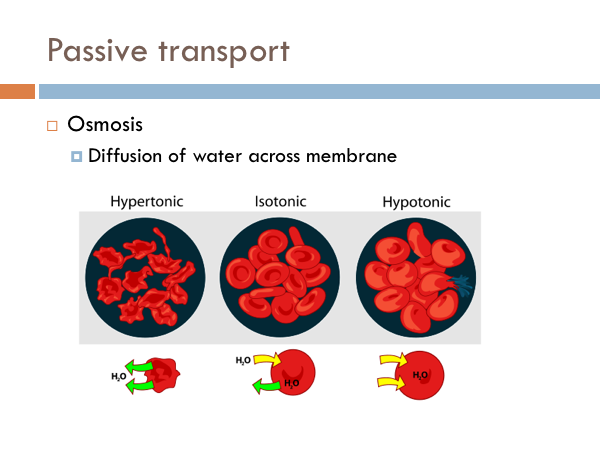
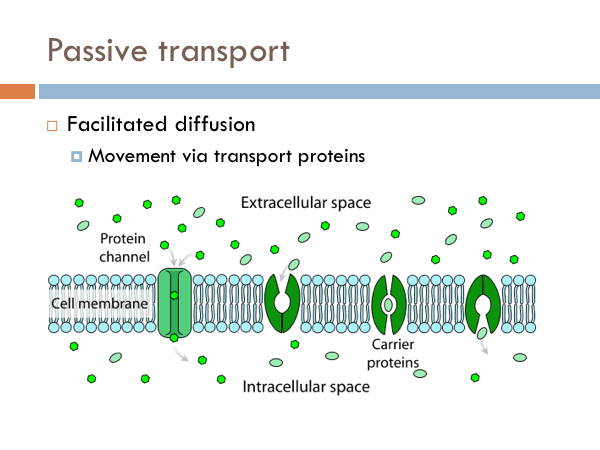
To the right, we have an example of a high concentration of molecules on the outer part of the cell membrane. Inside is a low concentration. The molecules in this example move across the membrane freely until the concentrations on both sides equalize. Water does this in cells. And we have a special term for simple diffusion of water. And that term is osmosis. Here we have three different types of osmotic conditions. A regular healthy cell is said to be isotonic. If you eat too much salt, you create a condition in which there are more salt molecules in the space between your cells that in your cells, and water will leave the cells creating a hypertonic condition. On the flip side, it is actually possible to die from drinking water. That’s right, it is possible to die from drinking too much water. Marathon runners have to be acutely aware of their water intake because the tendency is to drink water to cool down. There have been incidences where marathon runners have consumed too much water to the point where their cell become so hypotonic that they burst. But you shouldn’t worry too much it is pretty hard to do. Facilitated diffusion is another type of passive transport. Certain chemicals are too big to fit through the pores of the cell membrane. However, they are allowed to move from high concentration to low concentration through proteins. Here we have two different examples of how bigger materials move through the cell membrane with the help of proteins. On the left, we have a protein channel, Anything that can fit in that channel can move freely into and out of the cell. On the right, we have carrier proteins. These proteins have a specific shape that is the complement of certain molecules. Just like a lock and key. Once that key fits in that lock, the protein changes shape and releases the molecule to the other side of the membrane. Think of it like a revolving door.
Active Transport
Active transport requires energy to move substances from one side of the membrane to the other. Primary active transport uses ATP directly to transport molecules from one side of the membrane to the other. The classic example of this is the sodium-potassium pump. There are these cup shaped proteins that bind specifically with sodium ions. ATP comes by release energy which causes the shape of the cup to invert, forcing the sodium ions to get spit out the other side of the membrane. This is important because sodium is not very useful to a cell and it needs to get rid of it. On the flip side, Potassium is needed by the cell and has to be pumped in. But the interesting thing is that it pumped in by the same proteins. However, to get in doesn’t require any more energy. The protein is like two sea-saws working in sync. Right after the sodium gets spit out, the potassium binds to the protein and gets forced into the cell. This produces a cell with the insides of a high potassium concentration and a low sodium concentration.
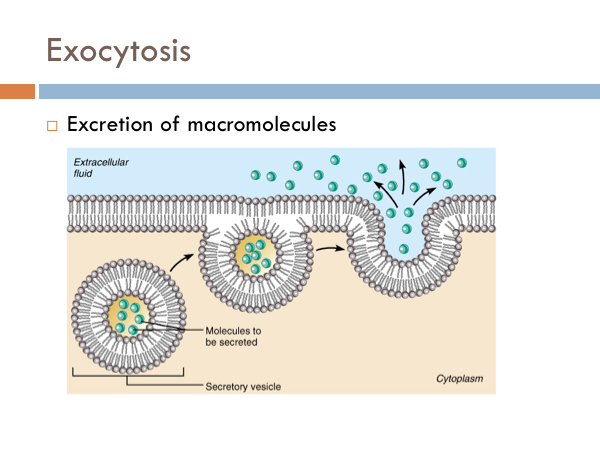
Exocytosis
Exocytosis. Within the cell, certain molecules that have no use to the cell are imprisoned by a liposome (made up of a phospholipid bilayer) or a secretory vesicle. Secretory vesicles move towards the cell membrane. The phospholipids of the secretory vesicle fuse with the cell membrane, eventually releasing the molecules to the extracellular space, a process known as exocytosis.
Endocytosis
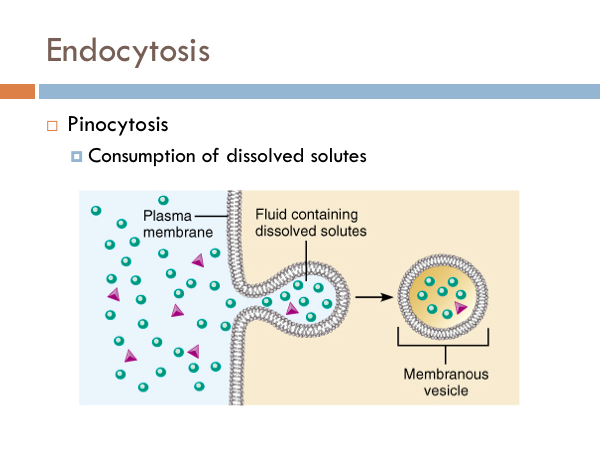
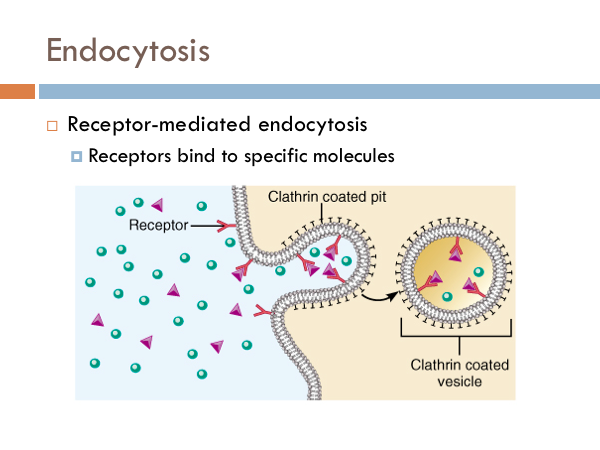
Endocytosis is the reverse of exocytosis, in which vesicles merge with a cell membrane depositing molecules. The simplest form of endocytosis is pinocytosis. Cell membranes can form cavities, which fill with particles outside the cell. The cell membrane pinches off the cavity into a vesicle and the phospholipid bilayer of the membranes attaches to itself producing a package of solutes that can float around the cell. Another type of endocytosis is receptor-mediated endocytosis. We said that the cell membrane is embedded with proteins. Some of these proteins from receptors. These receptors are like outfielders are to baseballs. They snatch them up. When those receptors fill, they cause a chemical reaction which causes the cell membrane to pinch in and eventually form a vesicle that floats around inside the cell. The advantage of this over pinocytosis is that specific chemicals are selected (hand-picked, if you will) to go into the cell. Where, in the case of pinocytosis, any solute that is there will make it into the cell.
The first cell
It is thought the first cell developed 4.0-4.3 billion years ago. That is nearly, right after the Earth came to be!!! There are several theories about how it came to be. The first cells could have blasted onto earth by meteorites, or spontaneously generated from deep-sea vents or by lightning. It is assumed that RNA was the first self-replicating molecule, and that the first cells were heterotrophs. In other words, they could not make their own energy. They were reliant on other energy sources. Plants, in contrast, make their own energy and are consider to be autotrophs. Humans are heterotrophs. We can produce our own food. We are reliant on plants. The cell theory of biology states that cells are the basic units of life. And there can not be cells with out cell membranes. The first cells probably included two elements a cell membrane enclosing RNA. Those two components are fundamental. In water, phospholipids spontaneously form bilayered vesicles. This could have preceded the genesis of RNA (or not). They very well could have arisen independently, and merged into a beautiful marriage, which evolved into what we now know as life.