Module Instructions: Atoms and Molecules
Pre-Lab: Atoms and Molecules
Watch the Atoms and Molecules pre-lab video to give you the background information on how to complete the lab.
Lab: Introduction to the Biochemistry
Materials
You will not need any lab materials for this activity. Complete the lab using the information provided in the lab text along with what you learned from the lecture.
Lab Tips
Below you will find suggestions for the most common mistakes in this lab. Use this as a guide. If you have any questions, feel free to reach out to me for guidance.
Exercise A: Drawing the Bohr Model of Atoms
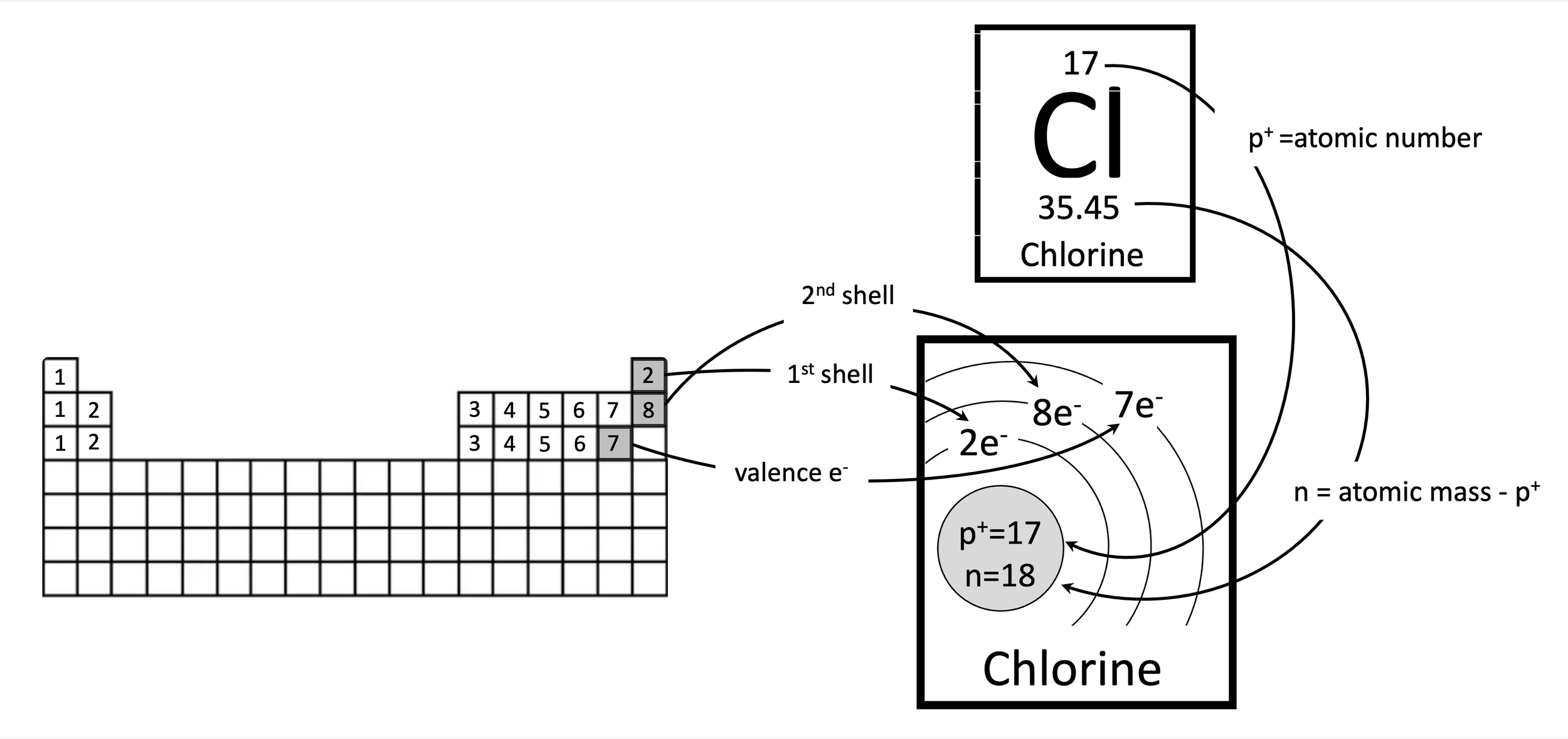
Problem 1. How to create a Bohr model for chlorine. The number of protons is equal to the atomic number. The number of electrons is equal to the number of protons to balance out the charge. The number of neutrons is the rounded atomic mass minus the number of protons. Since chlorine is in the third period (row), it will have three electron shells. The first and second shells are full, with two and eight electrons, respectively. That leaves seven electrons left over in the outer shell, the valence shell.
Exercise B: Ionic Bonds
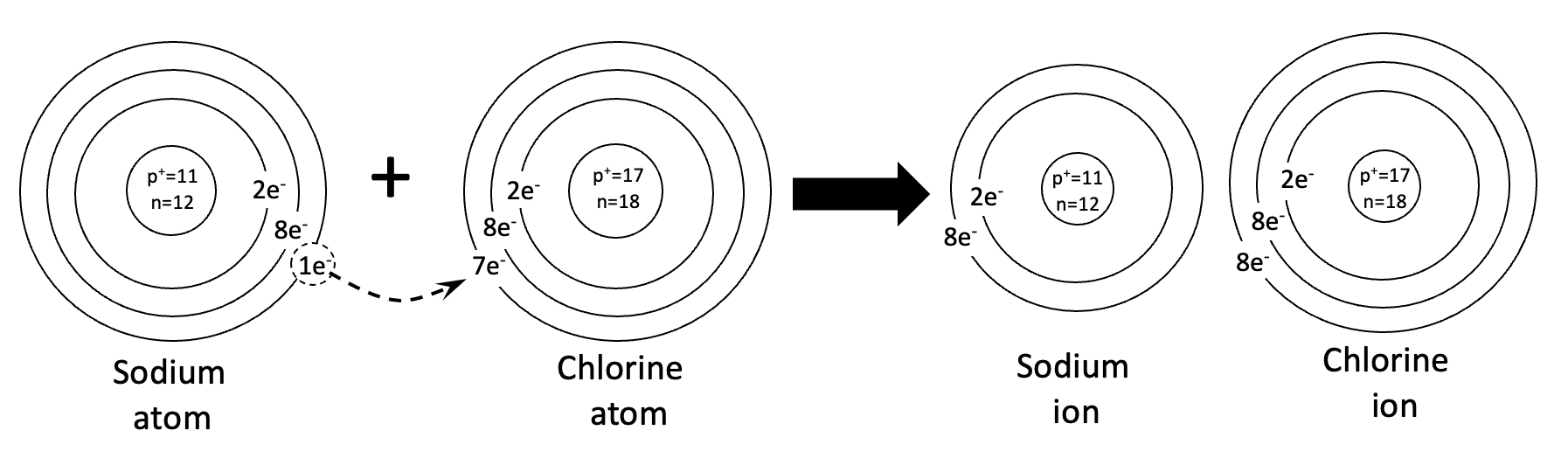
Problem 2. How to calculate the charge of a sodium atom. When calculating the charge, you must subtract the number of electrons from the number of protons. This is because protons have a positive charge and electrons have a negative charge. Neutrons have no charge. So they are not considered when calculating charge. Below is the work for the Chlorine atom. There are 17 protons and 17 electrons. So the charge is +17-17=0. There is no charge, or a neutral charge.
Problems 3 and 4. How to draw ionic bonding. For problems 3 and 4, be sure to use the template as a guide. For problem 4, please note that there are two atoms of chlorine in this reaction. Note that the ions have a different number of protons and electrons. That is what gives them an electromagnetic charge.
Exercise D: Covalent Bonds
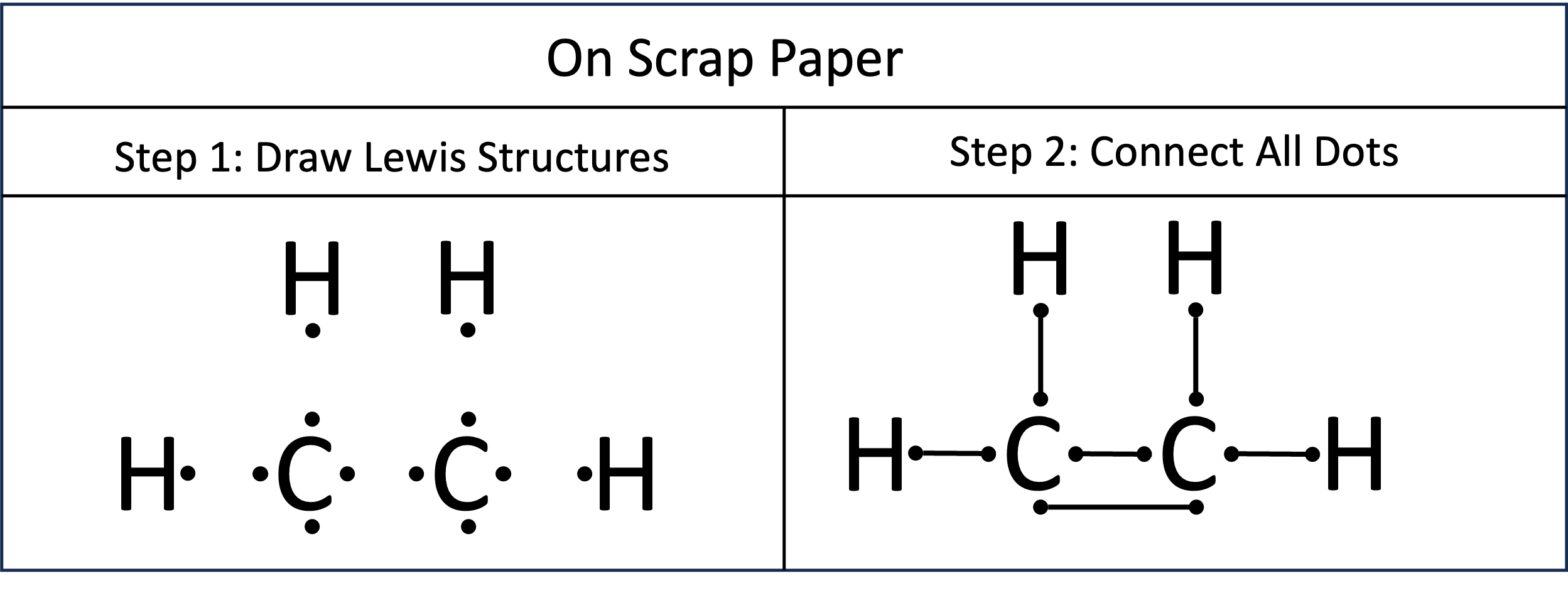
Problem 6. Preparation for creating Lewis Structures of C₂H₄. Step 1: On a scrap piece of paper, draw Lewis dot structures of all of the atoms. In this example there are two atoms of carbon and four of hydrogen. Where you place them doesn’t really matter. Step 2: Connect the dots. There are two rules. Rule 1: You can not connect dots from the same atom. Rule 2: You can only pair reactive electrons. These are the unpaired electrons in the Lewis Dot structure. Paired electrons do not undergo covalent bonding.
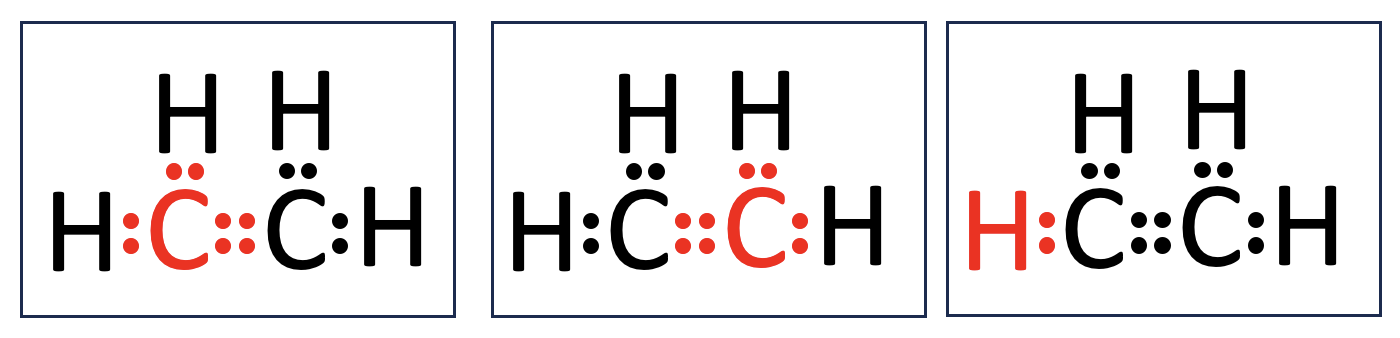
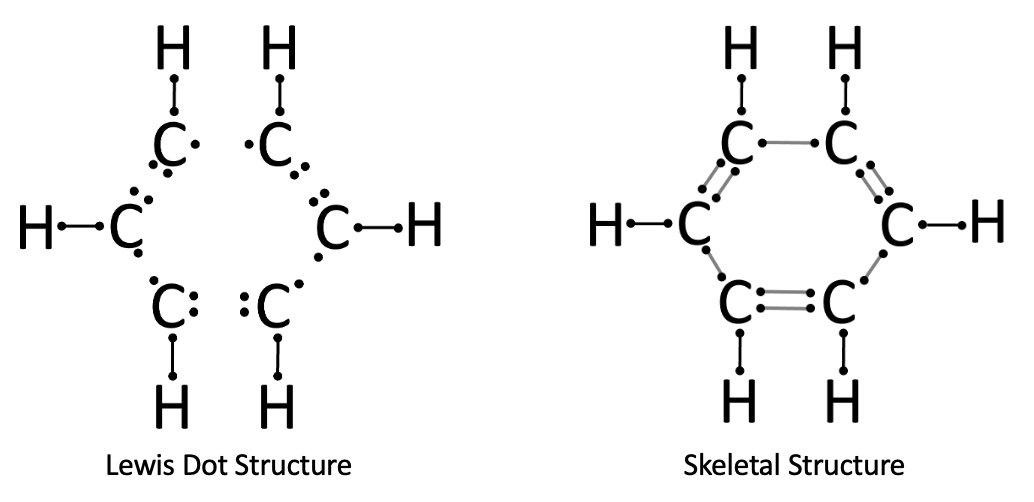
Problem 6. Lewis Dot Diagram of C₂H₄. From our scrap paper, we can see there are two electrons being shared between each carbon and hydrogen. In the Lewis Dot Diagram we would show this as two dots ( : ) between the atoms to represent a single covalent bond. The two carbons are sharing four atoms, known as a double bond. We show this as four dots ( : : ). Skeletal Structure of C₂H₄. For every two electrons shared between atoms, we visualize this as a bar. Single bonds are represented one bar ( - ). Double bonds are represented by two bars ( = ).
Problem 6. Check your work. Carbon atoms are in the second period of the periodic table. Atoms in the second (and third) periods can hold up to 8 valence electrons. By forming covalent bonds, they achieve a more energetically stable configuration. For example, a carbon atom is typically shown surrounded by 8 dots in a Lewis structure, representing this stability. Hydrogen, on the other hand, is in the first period. Because its electron shell can only hold 2 valence electrons, a hydrogen atom can form only one covalent bond with a neighboring atom.
Problem 7. Set up for C₆H₆. Benzene (C₆H₆) can be thought of as six repeating units, each consisting of one carbon atom bonded to one hydrogen atom. If you draw these six subunits in a row and try to connect the carbons, you will notice that one of the bonds becomes too long. Such a bond is not possible in a covalent structure, since atoms must remain close together. Instead, the molecule bends and the carbons connect in a ring, forming a hexagon.
Problem 7. Lewis Dot Structure and Skeletal Structure for C₆H₆. The bends in the covalent bonds creates a hexagonal shape, as we see in the resulting Lewis Dot Structure and Skeletal Structure.
Problem 7. Setup for glucose, (C₆H₁₂O₆). Using the same strategy as with benzene, you can think of C₆H₁₂O₆ as being composed of six subunits of C₁H₂O₁. If you draw six of these subunits and connect the reactive electrons, you can begin to see how the larger molecule forms. Keep in mind that each oxygen atom has two pairs of nonbonding (unreactive) electrons, which do not participate in covalent bonding.
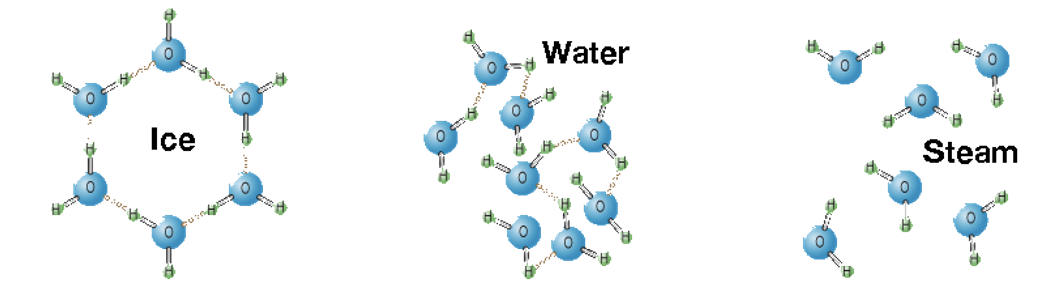
Problem 8. Hydrogen bonding in H₂O. This one is a bit tricky to visualize with just the wording in the lab. You can use this image as a guide.
Create a PDF
When finished, combine your work into one single PDF. Be sure you have written your name on the top of the first page. In the Start Here module, you’ll find a link with instructions on different ways to create a PDF.
Submit your Lab
After you’ve created the PDF, submit it to the Lab Submission link in the module.
Labs are due Friday at 5 PM. I will grade them early the following week and provide feedback in the comment box of the Lab Submission link.
Post-Lab: Atoms and Molecules
Complete and submit the Post-Lab in Canvas by Friday at 5 PM on the date listed in the course calendar. Be sure to check the calendar carefully so you don’t miss the deadline. Quizzes must be completed in a single sitting, and once you submit, you cannot reopen them. Late quizzes lose 10% per day, so plan ahead to avoid penalties. Make sure you have a reliable internet connection before starting the quiz.