Monosaccharides
Carbohydrates are sugars that can be made up of one or more monosaccharides. Sugars are widely variable in chemical structure and form, but all are made up of carbon (C), hydrogen (H) and oxygen (O). There are several different monosaccharides, but they all have proportionate amounts of carbon, hydrogen and oxygen (1C:2H:1O). For example glucose is a hexose sugar, which has 6 carbon atoms, 12 hydrogen and 6 oxygen, written as the molecular formula (C6H12O6). In other words, all monosaccharides follow the expression n(C1H2O1).
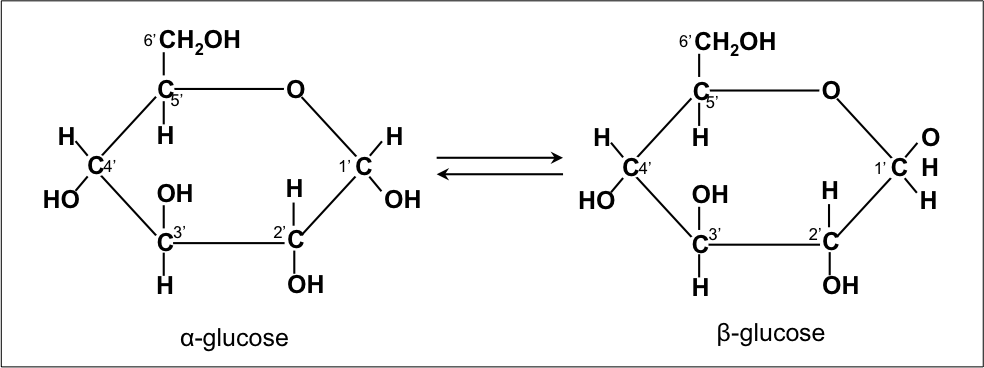
Two isomers of glucose: α-glucose and β-glucose (C6H12O6)
While monosaccharides can vary by the number of carbons available, there can be several different physical arrangements of sugars with the same molecular formula. Molecules with the same molecular formula but various spatial arrangements of atoms are known as isomers. For example, glucose is a hexose sugar (a monosaccharide containing 6 carbons, C6H12O6). In an aqueous solution, glucose develops into a ringed molecule which can orient in two different ways: α-glucose and β-glucose.
The carbons of sugars are numbered in a specific manner. The carbon to the clockwise direction of the oxygen held within the ring structure of glucose is identified as the 1’ carbon. The carbon clockwise of the 1’ carbon is the 2’ carbon, and so on. The two glucose monomers differ in their orientation of the hydroxyl (OH) and the hydrogen (H) attached at the 1’ carbon. In α-glucose, the hydrogen is closer to the oxygen held within the ring and in β-glucose the hydroxyl group is closest to the oxygen. While both isomers of glucose exist in equilibrium, β-glucose is slightly more stable.
Glucose is used directly by cells in the process of cellular respiration or fermentation. The energy held within chemical bonds of glucose is released through a series of reactions, which is used in the synthesis of adenosine triphosphates (ATPs). ATP is a molecule with high potential energy that when released can be used by all living organisms to do cellular work. ATP is the universal energy currency of the cell used by all known organisms. When cells have an excess of glucose, they can bond glucose molecules together for short- or long-term storage by covalent bonds known as glycosidic linkages.
Glycosidic Linkages and Disachharides
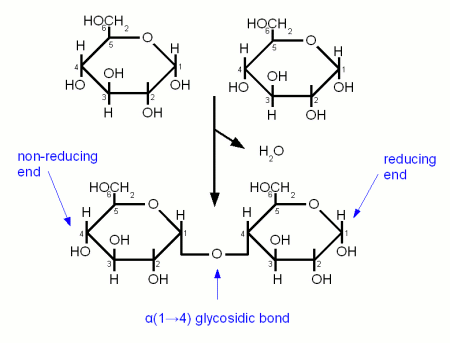
When an excess of glucose is present in cells, the monosaccharides bind together via covalent bonds, known as glycosidic linkages. A glycosidic linkage forms from a condensation reaction between two hydroxyl (OH) groups between any two monosaccharides. Two α-glucose molecules form a glycosidic linkage generating a disaccharide, known as maltose. The hydroxyl (OH) group attached to the 1’ carbon of one α-glucose reacts with a hydroxyl group attached to the 4’ carbon of a second hydroxyl group. One of the oxygens remains and forms a bond between the two α-glucose molecules, forming the disaccharide maltose. The hydrogen (H) and hydroxyl (OH) group that are removed from the sugars form a covalent bond creating water (H2O). This type of bond is known as an α-1,4-glycosidic linkage.
Storage Polysaccharides
When the cell requires more glucose, maltose can be broken back down into its two α-glucose components, which are then capable of undergoing cellular respiration or fermentation. However, when cells have excess glucose, they form storage polysaccharides, long chains of glucose bound together by glycosidic linkages.
Starch is a storage polysaccharide generated by plants that are made entirely of α-glucose. Starch can be stored by plants for days to years. There are two variants of starch: amylose and amylopectin. Amylose is unbranching polysaccharide of glucose linked together via α-1,4-glycosidic linkages. Amylopectin is a glucose polysaccharide that branches about every 30th monomer. Branching in amylopectin occurs between the 1’ carbon of one glucose and the 6’ carbon of an adjacent glucose.
Glycogen is a storage polysaccharide generated by animals, which is stored in their muscles and the liver. Glycogen is a short term storage molecule, lasting hours to days. Like starch, glycogen can be broken back down into glucose monomers which are capable of undergoing cellular respiration. Like amylopectin, glycogen is a branching polysaccharide of α-glucose. However, glycogen branches about every 10th monomer.
Structural Polysaccharides
Organisms can link monosaccharides to form sheets of polysaccharides useful in enhancing structural integrity. These structural polysaccharides are common in many organisms from bacteria to plants and animals. Plants link together β-glucose monomers producing the structural polysaccharide, cellulose. Glycosidic linkages form between adjacent β-glucose monomers forming linear chains. Adjacent linear chains connect to each other via hydrogen bonds between oxygen of the 2’ carbon of a β-glucose on one chain with a hydrogen attached to the 6’ carbon of an adjacent chain. These linear chains link with each other forming sheets.
Chitin is a polysaccharide found in animals and the cell walls of fungi. Chitin forms the exoskeleton of insects and crustaceans and is found in the hair and fingernails of humans. Like cellulose, it forms sheets of polysaccharides linked together via hydrogen bonding. Unlike cellulose, the monomer of chitin is different and is known as N-acetyl-glucosamine (NAG).
Bacteria have a cell wall made up of another type of structural polysaccharide, peptidoglycan. The monomer of peptidoglycan is similar to NAG. However, peptidoglycan differs from chitin in how the bonds are formed. While chitin forms hydrogen bonds between parallel polysaccharide chains, peptidoglycan have an additional chain of four amino acids to one of their carbons. The four amino acids on one monomer form a covalent bond (known as a peptide bond) with a group of four amino acids on another monomer on a parallel polysaccharide chain.
Lab: Benedict's Test for Sugar
Benedict’s test for sugars is a simple assay that detects the concentration of reducing monosaccharides in a solution. Some disaccharides are detectable by Benedict’s test, but sucrose (table sugar) is an unreactive disaccharide. Benedict’s solution is a reagent, a chemical that changes color in the presence of another chemical. In this test, you will have two control groups. A positive control is a substance known to have the chemical you are attempting to detect. You will use 4% glucose as your positive control. A negative control is a substance known to be absent of the chemical you are attempting to detect. Water is typically used as a negative control. In this assay, you will test three experimental groups with unknown sugar concentrations.
Lab: Amyoplasts
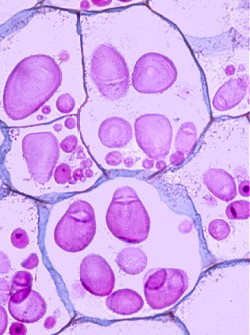
Amyoplasts are specialized organelles in plant cells that store starch. They are common in potato cells, which you will look at in this lab. Iodine is a reagent turns starch dark purple to black. Locate the amyoplasts in a potato cell.
Amyoplasts appear dark purple when stained iodine.